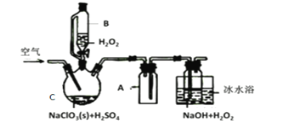
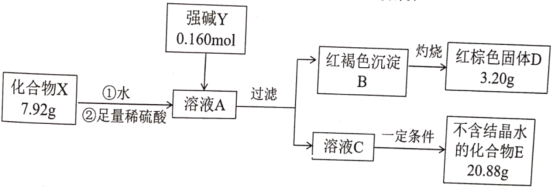
��Ŀ����
����Ŀ������ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni(OH)2��̼�ۡ���������Ϳ�����������Ƴɡ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о������ʵ���������£�
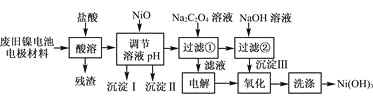
��֪����NiCl2������ˮ��Fe3����������Ni2����
����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O4��2H2O
��ij�¶���һЩ�������������Ksp����������������pH���±���ʾ��
|
| ��ʼ����pH | ������ȫpH |
|
|
|
|
|
|
|
|
|
|
|
|
�ش��������⣺
(1) ��NiO������Һ��pH����������������________�ͳ�����__________(�ѧʽ)��
(2) д������Na2C2O4��Һ�ķ�Ӧ�Ļ�ѧ����ʽ��_____________________��
(3) ��������Һʱ��������������ķ�����___________________________��
(4) д������������Ӧ�����ӷ���ʽ��___________________________________��
(5) ��μ���Ni(OH)3��ϴ�Ӹɾ���_______________________��
���𰸡�Fe(OH)3 Al(OH)3 NiCl2��Na2C2O4��2H2O=NiC2O4��2H2O����2NaCl ��ʪ��ĵ��۵⻯����ֽ 2Ni(OH)2��2OH����Cl2=2Ni(OH)3��2Cl�� ȡ���һ��ϴ��Һ������AgNO3��Һ�����а�ɫ�������ɣ������δϴ�Ӹɾ�����û�а�ɫ�������ɣ�֤��������ϴ�Ӹɾ�
��������
�Ͼ�����ص缫�����м�����������ʱ��Ni(OH)2��������������Ӧ����NiCl2��FeCl3��AlCl3������Ϊ̼�ۣ����ݱ������ݣ�����NiO������ҺpHʱ�����λ��Fe(OH)3��Al(OH)3��������֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O42H2O������Һ�м���Na2C2O4���NiC2O4��2H2O��NaCl��NiC2O4��2H2O�м���NaOHת��ΪNi(OH)2�����NaCl��Һ�õ�NaOH��H2��Cl2�����Cl2��Ni(OH)2������Ni(OH)3���ݴ˷�������
�Ͼ�����ص缫�����м�����������ʱ��Ni(OH)2��������������Ӧ����NiCl2��FeCl3��AlCl3������Ϊ̼�ۣ����ݱ������ݣ�����NiO������ҺpHʱ�����λ��Fe(OH)3��Al(OH)3����������Һ�м���Na2C2O4���NiC2O4��2H2O��NaCl��NiC2O4��2H2O�м���NaOHת��ΪNi(OH)2�����NaCl��Һ�õ�NaOH��H2��Cl2�����Cl2��Ni(OH)2������Ni(OH)3��
��1�����ݱ��п�ʼ�����ͳ�����ȫ��pH��Fe��OH��3��ʼ������pH=2.5��������ȫ��pH=2.9��Al��OH��3��ʼ������pH=3.4��������ȫ��pH=4.2�������IΪFe��OH��3������IIΪAl��OH��3��
��2����֪ʵ���¶�ʱ���ܽ�ȣ�NiC2O4��NiC2O4��H2O��NiC2O42H2O����NiCl2��Һ�м���Na2C2O4��Ӧ����NiC2O42H2O��NaCl����Ӧ�ķ���ʽΪ��NiCl2+Na2C2O4+2H2O�TNiC2O42H2O��+2NaCl��
��3����ҺΪ�Ȼ�����Һ������Ȼ�����Һʱ����������������ʪ��ĵ��۵⻯����ֽ����Cl2��
��4�����ˢڵõ�Ni��OH��2������Ȼ�����Һ����������Cl2��Ni(OH)2������Ni(OH)3����Ӧ�����ӷ���ʽΪ2Ni��OH��2+2OH-+Cl2�T2Ni��OH��3+2Cl-��
��5���������̣�����Ni(OH)3��ϴ�Ӹɾ�ֻҪ����ϴ��Һ�в���Cl-���ɣ�����Ϊ��ȡ���һ��ϴ��Һ��������������Һ�����а�ɫ�������ɣ���˵��δϴ�Ӹɾ������ް�ɫ������˵����ϴ�Ӹɾ���

����Ŀ�������£�������ĵ���ƽ�ⳣ�����£�
��ѧʽ | HF | HCN | H2CO3 |
���볣�� | Ka=3.5��10-4 | Ka=5.0��10-10 | Ka1=4.4��10-7 Ka2=4.7��10-11 |
��1��c(H+)��ͬ����������Һ��Ũ�ȴӴ�СΪ___��
��2����HCN��Һ����ʼŨ��Ϊ0.01mol��L-1��ƽ��ʱc(H+)ԼΪ__mol��L-1��ʹ����Һ��HCN�ĵ���̶�������c(H+)Ҳ����ķ�����__��
��3���к͵�����NaOH�����ĵ�pH�����������������ֱ�ΪaL��bL����a__(������������С������������������ͬ)b���к͵�Ũ�ȡ��������������������ҪNaOH�����ʵ���Ϊn1��n2����n1__n2��
��4����NaCN��Һ��ͨ��������CO2��������Ӧ�����ӷ���ʽΪ__��
��5�����ʵ��֤��������HCl��������__��