��Ŀ����
2����1�������ײ��ϡ��ǵ�����Ͽ�ѧ�о���ǰ�أ����о��ɹ��㷺Ӧ���ڴ������¿�ѧ�У���ν�����ײ��ϡ���ָ�о����������������ȴӼ���������ʮ���IJ��ϣ��罫���ײ��Ϸ�ɢ����ɢ���У����û������ܾ��е�������B��A����ȫ������Ĥ
B���ж����ЧӦ
C������Һ��ʽ�״
D����������һ��������Һ
��2���ѵ�����Һ���ڷ�ˮ�У��Ƴɵ��۽��壬�ش��������⣮
�ټ���ˮ��Һ�ͽ���������õķ�������һ���ɼ���ֱ�������ƿ��ɫҺ�壬�ɼ���һ������ͨ·��Ϊ���۽��壮
��60������ʱ���ڵ��۽����м������ø��װ���Ĥ���ϵ�����ڣ�������������ʢ������ˮ���ձ����ַ�Ӧ���Ӱ�Ĥ����������������ѿ�ǣ��ò�����������������
���� ��1���ɡ�����������ָ����ֱ���ڼ�������ʮ�IJ��ϣ����ɢ��Һ���ɢ���У���ɢ�ʵ�ֱ����1nm��100nm֮�䣻
��2������Ȼ�����ͬ��ˮ��Һ�ͽ����кܶ����ʲ��죬�����������������ɿ����������ʻ��Ƕ��������
�ڵ����ڵ���ø�������»�ˮ���С���ӣ�������Ĥ����ø��Ϊ���������ڰ�Ĥ���ڣ�
��� �⣺��1��ɢϵ�з�ɢ�ʵ�ֱ����1nm��100nm֮������ڽ����ɢϵ���ɡ�����������ָ����ֱ���ڼ�������ʮ���IJ��ϣ����ɢ��Һ���ɢ���У���ɢ�ʵ�ֱ����1nm��100nm֮�䣬��û�������ڽ��壬�������û������ܾ��е������ǽ�������ʣ�����ķ�ɢ�����ϴ���ͨ����Ĥ����������ֽ���嶼�ܲ��������ЧӦ���ʴ�Ϊ��B��
��2���ٽ������Һ�������ǣ�������ж����ЧӦ������Һ���߱������Ծݴ���������ߣ��ʴ�Ϊ����һ���ɼ���ֱ�������ƿ��ɫҺ�壬�ɼ���һ������ͨ·��Ϊ���۽��壻
�ڵ���ˮ�����������ǣ�������������Ĥ��������С���ӣ����������������ᴿ���壬�ʴ�Ϊ����ѿ�ǣ�������
���� ���⿼�齺��������Լ���Һ�ͽ���ļ����ᴿ֪ʶ���ѶȽ�С��ּ�ڿ���ѧ���Ի���֪ʶ��ʶ�ǣ�ע�����֪ʶ�Ļ������գ�

 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�| A�� | ��״���£�11.2LHCl��11.2LNH3��ֻ�Ϻ��еķ�����ΪNA | |
| B�� | 0.1mol �к���̼̼˫������ĿΪ0.4NA �к���̼̼˫������ĿΪ0.4NA | |
| C�� | ���³�ѹ�£�1molNO2��ˮ��Ӧ����Һ��NO3-����ĿΪNA | |
| D�� | 1molMg������������Ӧ����MgO��Mg3N2��ʧȥ�ĵ�����Ϊ2NA |
 ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka=5.6��10-11 | Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��������Һ��a��Na${\;}_{{2}_{\;}}$CO3 b��NaHCO3 c��NaClO d��CH3COONa�����ǵ�pH�ɴ�С���е�˳����a��c��b��d�����ţ���
��2�������£�0.1mol•L-1CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����BC��
A��c��H+��B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$C��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ D�� c��H+��•$\frac{c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
��3��CH3COOH��һԪ��HX����Һ��Ϊ100mL��pH=2����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����ͬ�¶�ʱCH3COOH�ĵ���ƽ�ⳣ��С�ڣ�����ڡ�����С�ڡ����ڡ��� HX�ĵ���ƽ�ⳣ����
��4��25��ʱ��CH3COOH��CH3COONa�Ļ����Һ�������pH=6������Һ��c��CH3COO-��-c��Na+��=9.9��10-7mol•L-1��������λ��Ч���֣���
����ԭ��Һ�еμ�������ˮ�������ݲ�������Һ�ʳȻ�ɫ��
����ʳȻ�ɫ����Һ�еμ�BaCl2ʱ�������ɣ�
�۳Ȼ�ɫ��Һ����ʹ���۱�������������Һ��һ�������ڵ������ǣ�������
| A�� | NH4+��Br-��CO32- | B�� | NH4+��I-��SO32- | C�� | Fe2+��I-��SO32- | D�� | Fe2+��Br-��CO32- |
 ��������ƣ�Na2S2O3���������մ����������Լ�����������ˮ�������ھƾ������ȡ������ֽ⣮��ҵ�Ͽ�����Ʊ�����Ӧԭ����
��������ƣ�Na2S2O3���������մ����������Լ�����������ˮ�������ھƾ������ȡ������ֽ⣮��ҵ�Ͽ�����Ʊ�����Ӧԭ����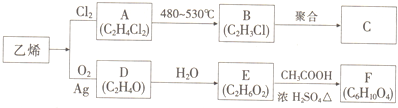


 ��
��
 ��
�� ��
�� ��
��