��Ŀ����
��15�֣�PCl3�����ڰ뵼�����������ӡ���ɢ�����й����ʵIJ����������£�
��һ���Ʊ�
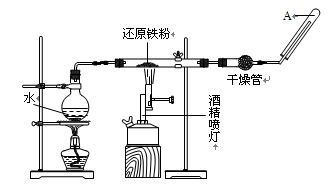
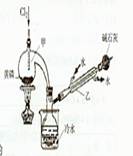
��ͼ��ʵ�����Ʊ�PCI3��װ�ã�����������ʡ�ԣ�
��1�������ҵ����� ��
��2��ʵ�����Ʊ�Cl2�����ӷ���ʽ�� ��
��3����ʯ�ҵ������� �� ��
��4������������ͨ�˸���Cl2֮ǰ��Ӧ��ͨ��һ��ʱ���CO2����Ŀ���� ��
�������ᴿ
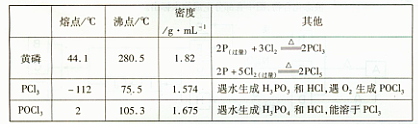
�ֲ�Ʒ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ��____����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
����������
�ⶨ��Ʒ��PCl3���ȵķ������£�Ѹ�ٳ�ȡm,g��Ʒ��ˮ����ȫ�����500mL��Һ��ȡ��2 5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ��
5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ��
��֪��H3PO3+H2O+I2=H3PO4+2HI��I2+2Na2S2O3=2NaI+Na2S4O6������ⶨ������û��������Ӧ��
�����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ�����ػ���
��һ���Ʊ�
��ͼ��ʵ�����Ʊ�PCI3��װ�ã�����������ʡ�ԣ�


��1�������ҵ����� ��
��2��ʵ�����Ʊ�Cl2�����ӷ���ʽ�� ��
��3����ʯ�ҵ������� �� ��
��4������������ͨ�˸���Cl2֮ǰ��Ӧ��ͨ��һ��ʱ���CO2����Ŀ���� ��
�������ᴿ
�ֲ�Ʒ�г�����POCl3��PCl5�ȡ���������ȳ�ȥPCl5��ͨ��____����ʵ��������ƣ������ɵõ�PCl3�Ĵ�Ʒ��
����������
�ⶨ��Ʒ��PCl3���ȵķ������£�Ѹ�ٳ�ȡm,g��Ʒ��ˮ����ȫ�����500mL��Һ��ȡ��2
 5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ��
5.00mL���������c1mol��L-lV1mL����Һ����ַ�Ӧ������c2mol��L-1Na2S2O3��Һ�ζ������ĵ⣬�յ�ʱ����V2mLNa2S2O3��Һ����֪��H3PO3+H2O+I2=H3PO4+2HI��I2+2Na2S2O3=2NaI+Na2S4O6������ⶨ������û��������Ӧ��
�����������ݣ��ò�Ʒ��PCl3����������Ϊ ���ú���ĸ�Ĵ���ʽ��ʾ�����ػ���

��һ����1�������ܣ�����ȴ�������ã���2������MnO2����Ũ������Cl2����3����ʯ
�ҵ�����һ��������Cl2����ֹ��Ⱦ������һ�����ֹ�����е�ˮ�����ʹPCl3ˮ�⣻��4��
��PCl3�ɱ�������������ˮ��Ӧ����������װ��Ҫ��������ˮ�����������ݱ��е���Ϣ����
ͨ������ķ�����ȥPCl3�е����ʣ�
���������ݹ�ϵʽPCl3~H3PO3~I2����m��PCl3��=137.5x(c1V1-1/2c2V2)x500/25x10-3��
�ҵ�����һ��������Cl2����ֹ��Ⱦ������һ�����ֹ�����е�ˮ�����ʹPCl3ˮ�⣻��4��
��PCl3�ɱ�������������ˮ��Ӧ����������װ��Ҫ��������ˮ�����������ݱ��е���Ϣ����
ͨ������ķ�����ȥPCl3�е����ʣ�
���������ݹ�ϵʽPCl3~H3PO3~I2����m��PCl3��=137.5x(c1V1-1/2c2V2)x500/25x10-3��

��ϰ��ϵ�д�
�����Ŀ
 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�
 NaCl���ʣ�Ϊ��
NaCl���ʣ�Ϊ��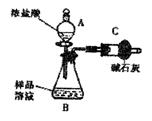
 ���ظ�ʹ�ã��г������ԣ�
���ظ�ʹ�ã��г������ԣ�