��Ŀ����
18����ͼ����Ϊ�����ᡢ��������ķ��ӽṹ�����ʯ��CuO�ľ����ṹ��
��ش��������⣺
��1����̬Cuԭ�ӵļ۵����Ų�ʽ��3d104s1������ͼ����������Ԫ�أ���һ�����ܱ�ͬ������������Ԫ�ض�С��Ԫ����O��S��
��2�������й������ᡢ��������������У�������Ǣڣ�
������������к��ЦҼ����м���Ŀ֮��Ϊ3��2
������������У���Ԫ����̼Ԫ�ص�ԭ�Ӿ�ΪSP�ӻ�
�����������������ụΪͬ���칹��
���������������������ߵķе�Ƚϣ���������ķе�ϸ�
��3��SCN-���ӳ������壬�����Fe3+��SCN-���������������ԭ����S��N��
��4��������ʯ���������ڵ�̼ԭ�ӽ������У�����ʯ����ͼ�пռ�������34%��������λ��Ч���֣���$\sqrt{3}$=1.732��
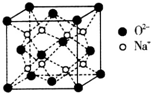
��5��CuOΪ�ķ�������a=b��c����=��=��=90�㣩ͼ�кڵ����������д��������ͭ�����������λ��Ϊ4��CuO���ܶ���6.6g•cm-3��������λ��Ч���֣�
���� ��1��Cuԭ�Ӻ��������Ϊ29�������������ԭ����д��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�����A�塢��A��ֱ�Ϊȫ���������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�
��2���ٵ���Ϊ�Ҽ���˫������1���Ҽ���1���м�����
������������У�S��Cԭ�Ӿ��γ�2���Ҽ���Sԭ�Ӻ���2�Թµ��Ӷԣ���̼ԭ��û�й¶Ե��ӣ���ԭ���ӻ������ĿΪ4��Cԭ���ӻ������ĿΪ2��
�۷���ʽ��ͬ���ṹ��ͬ�Ļ����ﻥΪͬ���칹�壻
�������������֮���γ���������������֮�䲻���γ������
��3�����������ԭ�Ӻ��й¶Ե��ӣ�
��4�����ݾ�̯�����㾧����Cԭ����Ŀ����̼ԭ��ֱ��Ϊa�����㾧����Cԭ���������̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ������ߴ�����Խ����ϣ���Ϊ�Խ��߳���$\frac{1}{4}$������Խ��߳�Ϊ4a�����ⳤΪ$\frac{4\sqrt{3}}{3}$a�������Ϊ��$\frac{4\sqrt{3}}{3}$a��3���ټ��㾧������������ռ�������=$\frac{ԭ�������}{�������}$��100%��
��5��Cuԭ�Ӱ뾶������ԭ�Ӱ뾶��CuO��Cu��Oyԭ����λ����ȣ����ݾ�̯�����㾧����Cu��Oԭ����Ŀ����ʾ�������������ٸ��ݦ�=$\frac{m}{V}$���㣮
��� �⣺��1��Cuԭ�Ӻ��������Ϊ29���۵����Ų�ʽ��Ϊ3d104s1��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�����A�塢��A��ֱ�Ϊȫ���������ȶ�״̬����һ�����ܸ���ͬ��������Ԫ�صģ�����������Ԫ�أ���һ�����ܱ�ͬ������������Ԫ�ض�С��Ԫ����O��S��
�ʴ�Ϊ��3d104s1��O��S��
��2���ٵ���Ϊ�Ҽ���˫������1���Ҽ���1���м���������������к��ЦҼ����м���Ŀ֮��Ϊ3��2������ȷ��
������������У�S��Cԭ�Ӿ��γ�2���Ҽ���Sԭ�Ӻ���2�Թµ��Ӷԣ���̼ԭ��û�й¶Ե��ӣ���ԭ���ӻ������ĿΪ4��Cԭ���ӻ������ĿΪ2��Sԭ�Ӳ�ȡsp3�ӻ���Cԭ�Ӳ�ȡsp�ӻ����ʴ���
���������������������ʽ��ͬ�����߽ṹ��ͬ����Ϊͬ���칹�壬����ȷ��
�������������֮���γ���������������֮�䲻���γ����������������ķе�ϸߣ�����ȷ��
�ʴ�Ϊ���ڣ�
��3��SCN-������Sԭ�ӡ�Nԭ�Ӿ����й¶Ե��ӣ�������������ԭ�ӣ�
�ʴ�Ϊ��S��N��
��4��һ�������к�̼ԭ����Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8����̼ԭ��ֱ��Ϊa��������Cԭ�������=8��$\frac{4}{3}$�У�$\frac{a}{2}$��3��̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ������ߴ�����Խ����ϣ���Ϊ�Խ��߳���$\frac{1}{4}$������Խ��߳�Ϊ4a�����ⳤΪ$\frac{4\sqrt{3}}{3}$a�������Ϊ��$\frac{4\sqrt{3}}{3}$a��3�������ռ�������={[8��$\frac{4}{3}$�У�$\frac{a}{2}$��3]�£�$\frac{4\sqrt{3}}{3}$a��3}��100%��34%��
�ʴ�Ϊ��34%��
��5��Cuԭ�Ӱ뾶������ԭ�Ӱ뾶���ʺ�ɫ��ΪOԪ�أ�CuO��Cu��Oԭ����λ����ȣ���ɫ����λ��Ϊ4��������λ��Ϊ4��������Cuԭ������Oԭ����Ŀ��Ϊ4����������Ϊ4��$\frac{80}{6.02��1{0}^{23}}$g�����ܶ�Ϊ4��$\frac{80}{6.02��1{0}^{23}}$g��[��400��10-10cm��2��500��10-10cm]=6.6g•cm-3��
�ʴ�Ϊ������4��6.6��
���� �����Ƕ����ʽṹ�Ŀ��飬�漰��������Ų��������ܡ���ѧ���������ӻ���ʽ�����ӽṹ�����ʡ���������ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��4���м���Ϊ�״��㡢�ѵ㣬��Ҫѧ���߱�һ���Ŀռ���������ѧ����������

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�| A�� | A��B��C��D | B�� | C��D��A��B | C�� | D��A��B��C | D�� | A��B��D��C |
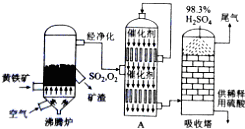
��1�����᳧����¯�ų��Ŀ����к���Fe2O3��CuO��CuSO4����CuO��SO2�ڷ���¯�л��϶��ɣ���CuFeS2�ǻ��������һ�ɷ֣�д������CuFeS2�Ļ�ѧ����ʽΪ4CuFeS2+13O2$\frac{\underline{\;����\;}}{\;}$ 4CuO+2Fe2O3+8SO2��
��2������ͼ���豸A�ǽӴ��ң����豸���ƣ������и��豸�ϲ�Ѹ��ʹ�õ�����ΪSO2��O2��
��3������������98.3%Ũ��������SO2��ԭ���ǿ��Է�ֹ�γ�������ʹ��������������ȫ��
��4�����ݹ�������ͼ�������Ƕ��ж�����˵����ȷ����ABDE������ţ�
A��Ϊʹ��������ȼ�գ��轫�����
B������¯���ų���¯���ɹ�����
C��ʹ�ô��������SO2�ķ�Ӧ���ʺ�ת����
D��β����SO2����NaOHŨ��Һ������
E�����᳧�ij�ַ��ѡ��������������Ĺ�ҵ���еĽ���
��5������¯�ų���¯���е�����ͭ���������������¯�¶Ȳ�ͬ���仯�����±���
| ����¯�¶�/�� | 600 | 620 | 640 | 660 |
| ¯����CuSO4����������/% | 9.3 | 9.2 | 9.0 | 8.4 |
��6��ij���᳧Ϊ�ⶨ�豸A������������SO2�����������ȡ280mL��������ɱ�״����������Ʒ������Fe��SO4��3��Һ��ȫ��Ӧ������������Ӱ�죩����Ũ��Ϊ0.02mol/L��K2Cr2O7����Һ�ζ����е㣬����K2Cr2O7��Һ25.00mL����Ӵ�������������SO2���������Ϊ12.00%��
| A�� | ���ʵķе㣺a��d��c | |
| B�� | �����ӵİ뾶��c��d��a��b | |
| C�� | a��b��c�����Ӷ����ƻ�ˮ�ĵ���ƽ�� | |
| D�� | a��b��d������������Ӧ��ˮ�������������ܷ�Ӧ |
| A�� | �ܱ�������1mol N2��3mol H2��ַ�Ӧ������ķ�����Ϊ2NA | |
| B�� | 2L 0.5 mol•L-1��������Һ�к��е�H+����Ϊ2NA | |
| C�� | �ڱ�״���£�22.4L��������ԭ����ĿΪ2NA | |
| D�� | 1molNa������O2��Ӧ������Na2O��Na2O2�Ļ�����ʧȥNA������ |
 H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺
H��C��N��O��Na��Fe��Cu�dz���������Ԫ�أ���ش��������⣺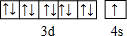 ��
��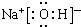 ��
��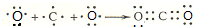
 ��
��
 $��_{Zn/H_{2}O}^{O_{3}}$
$��_{Zn/H_{2}O}^{O_{3}}$ +
+
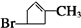 ��E
��E ��
��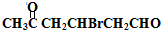 +2Cu��OH��2$\stackrel{��}{��}$
+2Cu��OH��2$\stackrel{��}{��}$ +Cu2O��+2H2O��
+Cu2O��+2H2O�� ��
��