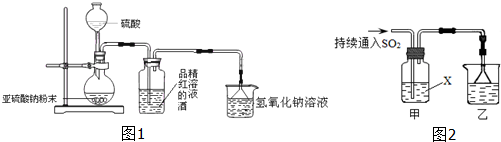
��Ŀ����
��A����ȩ B������ C�������� D���Ҵ� E������ F�������� G�����ᱵ�����л�����ѡ����ʵ����ʣ����������ڿո��ڣ�
��1���������� ��
��2����̼���Ʒ�Ӧ��CO2���ɵ��� ��
��3����װ�εļҾ���ɢ�������ж������� ��
��4��ͨ��������ӦΪ����������ṩ�������� ��
��5��ҽ���ϳ����������Ϊ75%�� ��Һ����������
��6�������л��߷��ӻ�������� ��
��7��ҽ���ϳ����������͡����� ��
��1����������
��2����̼���Ʒ�Ӧ��CO2���ɵ���
��3����װ�εļҾ���ɢ�������ж�������
��4��ͨ��������ӦΪ����������ṩ��������
��5��ҽ���ϳ����������Ϊ75%��
��6�������л��߷��ӻ��������
��7��ҽ���ϳ����������͡�����
���㣺�л���ѧ��Ӧ���ۺ�Ӧ��
ר�⣺�л���Ӧ
��������1������л���Ϊ���飻
��2������������ԣ����Ա�̼��ǿ��
��3������װ�εļҾ���ɢ�������ж������Ǽ�ȩ��
��4��������Ϊ����������ṩ������
��5���Ҵ�������ɱ��������
��6������л��߷��ӻ�����Ķ��������
��7�����ᱵ������ˮҲ�������ᣬ���������ͣ�
��2������������ԣ����Ա�̼��ǿ��
��3������װ�εļҾ���ɢ�������ж������Ǽ�ȩ��
��4��������Ϊ����������ṩ������
��5���Ҵ�������ɱ��������
��6������л��߷��ӻ�����Ķ��������
��7�����ᱵ������ˮҲ�������ᣬ���������ͣ�
���
�⣺��1������л���Ϊ���飬���ԭ��������С���ʴ�Ϊ��B��
��2���������Ե�ֻ�����ᣬ��̼���Ʒ�Ӧ�ų�������̼���ʴ�Ϊ��E��
��3����ȩ�����ڷ�����������װ�β����У��ʴ�Ϊ��A��
��4��������Ϊ����������ṩ�������ʴ�Ϊ��C��
��5���Ҵ���ʹ�����ʱ��ԣ�������ɱ���������ʴ�Ϊ��D��
��6��������Ϊ�߾����Է��������ϴ�Ϊ�߷��ӻ�����ʴ�Ϊ��F��
��7�����ᱵ������ˮҲ�������ᣬ���������ͣ��ʴ�Ϊ��G��
��2���������Ե�ֻ�����ᣬ��̼���Ʒ�Ӧ�ų�������̼���ʴ�Ϊ��E��
��3����ȩ�����ڷ�����������װ�β����У��ʴ�Ϊ��A��
��4��������Ϊ����������ṩ�������ʴ�Ϊ��C��
��5���Ҵ���ʹ�����ʱ��ԣ�������ɱ���������ʴ�Ϊ��D��
��6��������Ϊ�߾����Է��������ϴ�Ϊ�߷��ӻ�����ʴ�Ϊ��F��
��7�����ᱵ������ˮҲ�������ᣬ���������ͣ��ʴ�Ϊ��G��
���������⿼���Ϊ�ۺϣ��漰�л������ɡ��ṹ�����ʺ�Ӧ�õĿ��飬��Ŀ��Ϊ������ע�����֪ʶ�Ļ��ۣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
����˵���У���ȷ���ǣ�������
| A����ԭ�Ӻ������ӵĻ�ѧ������ͬ |
| B��ʪ����������Ư���� |
| C�������ж���������Ҳ�ж� |
| D�������������Ӷ��Ի���ɫ |
�����йؾ���������д�����ǣ�������
| A��ʯī�IJ�״�ṹ���ɹ��ۼ��γɵ���С��̼ԭ�ӻ���������̼ԭ�� |
| B���Ȼ��ƾ�����ÿ��Na+��Cl-��Χ���ڵ���6��Cl-��Na+ |
| C����CsCl������ÿ��Cs+��Χ���ڵ���8��Cl-������ÿ��Cs+�Ⱦ�����ڵ�Ҳ��8��Cs+ |
| D�������������ܶѻ��Ľ��������У�ÿ������ԭ����Χ���ڵ���4������ԭ�� |
��1molij���ʹ��ֳ����ȷݣ�����һ�ݳ��ȼ�պ�����1.5mol CO2����һ���������Ʒ�Ӧ����5.6L H2����״���������ִ����ӽṹ�г��ǻ��⣬�������ֲ�ͬ����ԭ�ӣ������ִ��ǣ�������
A��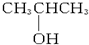 |
B��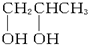 |
| C��CH3CH2CH2OH |
| D��CH3CH2OH |
�����йؾ���������У�����ȷ���ǣ�������
| A�����ʯΪ�ռ���״�ṹ���ɹ��ۼ��γɵ�̼ԭ�ӻ��ϣ���С�Ļ�����6��̼ԭ�� |
| B���Ȼ��ƾ����У�ÿ��Na+��Χ������ȵ�Na+����6�� |
| C���Ȼ�菉����У�ÿ��Cs+��Χ����8��Cl- |
| D���ɱ������У�ÿ��CO2������Χ����12��CO2���� |
��Li��Na��K��Rb��Cs�ı仯���ɲ����ϵ��ǣ�������
| A����ˮ���ᷴӦ�û����������� |
| B�������Ե�ǿ�� |
| C���۵� |
| D�����Ӳ��� |
���������У�����֤��ij��������һ���������Ӽ����ǣ�������
| A��������ˮ |
| B�����нϸߵ��۵� |
| C��ˮ��Һ�ܵ��� |
| D�����岻���磬������״̬�ܵ��� |
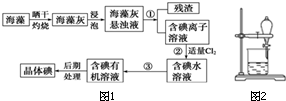 ����ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���������ͼ1��
����ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���������ͼ1��