��Ŀ����
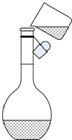 ʵ��������Ҫ����500mL0.10mol/L��NaOH��Һ���ش��������⣮
ʵ��������Ҫ����500mL0.10mol/L��NaOH��Һ���ش��������⣮��1������������ƽ����NaOH����
��2����������ɴ�ʵ�黹��Ҫ�������в���������ͷ�ιܡ�
��3���������Ƶ���Һ��ȡ��50mL�����Լ�ƿ�У������ϱ�ǩ����ǩ�ϵ�������
��4���������Ƶ���Һ��ȡ��10mL��Һ����ˮϡ����20mL�����ʱ�������ʵ���Ũ��Ϊ
��5�����в����������Ƶ���ҺŨ��û��Ӱ�����
A������ʱ�Ѿ��۲쵽NaOH������泱ʪ
B�����ձ����ܽ�����Һ����ע������ƿ�У�Ȼ������������ˮ���̶���
C��ҡ�ȶ��ݺ����ý�ͷ�ι�������ƿ�еμ�����ˮ���̶���
D��������Һǰ������ˮ��ϴ����ƿ����δ���
��6����ͼ��ijͬѧת����Һ��ʾ��ͼ��ָ��ͼ�д���֮����
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
��������1�����ݼ��㹫ʽm=cVM���������������Ƶ�������
��2����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡȡ������
��3����ǩ�ϵ�����Ϊ���ʵ����Ƽ���Һ�����ʵ���Ũ�ȣ�
��4��������Һϡ���������ʵ����ʵ�����������ϡ�ͺ���Һ�����ʵ���Ũ�ȣ�
��5������c=
�ж�������Һ�������nƫС��Cƫ����������Һ��Ũ��ƫ�ͣ����nƫ���VƫС����������Һ��Ũ��ƫ�ߣ�
��6������ת����Һʱ����ȷ���ڽ����жϣ����ܵ������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
��2����������һ�����ʵ���Ũ�ȵ���Һ�IJ���ѡȡ������
��3����ǩ�ϵ�����Ϊ���ʵ����Ƽ���Һ�����ʵ���Ũ�ȣ�
��4��������Һϡ���������ʵ����ʵ�����������ϡ�ͺ���Һ�����ʵ���Ũ�ȣ�
��5������c=
| n |
| V |
��6������ת����Һʱ����ȷ���ڽ����жϣ����ܵ������ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ�
���
�⣺��1��m=cVM=0.1mol/L��0.5L��40g/mol=2.0g���ռ�����ˮ�Ҿ���ǿ��ʴ�ԣ����Գ����ռ�ʱҪ�����ձ��У�
�ʴ�Ϊ��2.0��
��2������һ�����ʵ���Ũ�ȵ���Һ�����ǣ��������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ת�ơ����ݡ�ҡ�ȵȣ�ʹ�õ������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի���Ҫ����Ϊ��500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��3���������Ƶ���Һ��ȡ��50mL�����Լ�ƿ�У������ϱ�ǩ����ǩ�ϵ�����������������Һ����Ũ�ȣ�����0.10mol/L NaOH��Һ��
�ʴ�Ϊ��0.10mol/L NaOH��Һ��
��4���������Ƶ���Һ��ȡ��10mL��Һ����ˮϡ����20mL������c=
�����ʵ����ʵ������䣬��Һ�������Ũ�ȳɷ��ȣ�
����ϡ�ͺ���Һ��Ũ��Ϊ��0.10mol/L��
=0.05mol/L��
�ʴ�Ϊ��0.05mol/L��
��5��A���������Ƴ�������ʵ����ʵ���ƫС������c=
��֪��������Һ��Ũ��ƫ�ͣ���A����
B��NaOH�ܽ�ʱ�ų��������ȣ�δ��ȴ����������Һ��������Һ�����ƫС������c=
��֪��������Һ��Ũ��ƫ�ߣ���B����
C��ҡ�ȶ��ݺ����ý�ͷ�ι�������ƿ�еμ�����ˮ���̶��ߣ��������Ƶ���Һ���ƫ����c=
��֪��������Һ��Ũ��ƫ�ͣ���C����
D��������Һǰ������ˮ��ϴ����ƿ����δ��ɣ����ڶ��ݻ���Ҫ��������ˮ�����Բ�Ӱ�����ƽ������D��ȷ��
��ѡD��
��6��ת����Һʱ����ʹ�ò�����������������������Һ��������ƿ��ߣ�ʹ��Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��δ�ò�����������ʹ���ʵ����ʵ������ͣ�
�ʴ�Ϊ��2.0��
��2������һ�����ʵ���Ũ�ȵ���Һ�����ǣ��������ܽ⡢��ȴ��ת�ơ�ϴ�ӡ�ת�ơ����ݡ�ҡ�ȵȣ�ʹ�õ������У�������ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ����Ի���Ҫ����Ϊ��500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
��3���������Ƶ���Һ��ȡ��50mL�����Լ�ƿ�У������ϱ�ǩ����ǩ�ϵ�����������������Һ����Ũ�ȣ�����0.10mol/L NaOH��Һ��
�ʴ�Ϊ��0.10mol/L NaOH��Һ��
��4���������Ƶ���Һ��ȡ��10mL��Һ����ˮϡ����20mL������c=
| n |
| V |
����ϡ�ͺ���Һ��Ũ��Ϊ��0.10mol/L��
| 10mL |
| 20mL |
�ʴ�Ϊ��0.05mol/L��
��5��A���������Ƴ�������ʵ����ʵ���ƫС������c=
| n |
| V |
B��NaOH�ܽ�ʱ�ų��������ȣ�δ��ȴ����������Һ��������Һ�����ƫС������c=
| n |
| V |
C��ҡ�ȶ��ݺ����ý�ͷ�ι�������ƿ�еμ�����ˮ���̶��ߣ��������Ƶ���Һ���ƫ����c=
| n |
| V |
D��������Һǰ������ˮ��ϴ����ƿ����δ��ɣ����ڶ��ݻ���Ҫ��������ˮ�����Բ�Ӱ�����ƽ������D��ȷ��
��ѡD��
��6��ת����Һʱ����ʹ�ò�����������������������Һ��������ƿ��ߣ�ʹ��Һ�����ʵ����ʵ���ƫС�����Ƶ���ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��δ�ò�����������ʹ���ʵ����ʵ������ͣ�
���������⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ������������е��Ѷȵ����⣬���������ǿ�������߿��������������У�ע������ԣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ������˼ά�������Ͻ��Ĺ淶ʵ�����������������ѵ�������������

��ϰ��ϵ�д�
 ������������ϵ�д�
������������ϵ�д�
�����Ŀ
����ɫ��Һ���ܴ���������������ǣ�������
| A��Al3+��Cu2+��SO42-��Cl- |
| B��NH4+��Mg2+��SO42-��Cl- |
| C��Na+��HCO3-��K+��OH- |
| D��Mg2+��K+��Cl-��NO3- |
���ж��йػ�ѧ��Ӧ���̻�ʵ������Ľ����У���ȷ���ǣ�������
| A��Cl2��ˮ��Һ���Ե��磬˵��Cl2�ǵ���� |
| B���ڸ��������£�C���û���SiO2�е�Si��˵��C��������ǿ��Si |
| C������۵⻯�ص���Һ�м�����ˮ����Һ��Ϊ��ɫ��˵��Cl2��������ǿ��I2 |
| D���ڵ��з�̪��Na2CO3��Һ�У�����BaCl2��Һ����Һ��ɫ��˵��BaCl2��Һ������ |
��֪��NaHS��MgSO4��NaHSO3��ɵĻ�����Ԫ�ص�������Ϊa%������������Ԫ�ص���������Ϊ��������
| A��a% |
| B��2a% |
| C��1-1.75a% |
| D��1-0.75a% |
��NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ�������
| A��4 g��ˮ��D2O��������������Ϊ0.2NA |
| B��4.48 L H2��O2�Ļ������������������Ϊ0.2NA |
| C��0.2 mol Cl2�ܽ��ڵ������ˮ�У�ת�Ƶ�����Ϊ0.2NA |
D��12.6 g�����谷���ṹ����ͼ������̼����������ĿΪ0.6NA |


 ͬѧ��Ϊ��̽��þ�������ᡢ���ᷴӦʱ��Ũ�Ȼ��¶ȶԷ�Ӧ���ʣ��۲�þ����ʧ��ʱ�䣩��Ӱ�죬�����������»�ѧ��Ʒ��0.2mol/L��0.40mol/L��HCl��Һ��0.2mol/L��0.40mol/L��CH3COOH��Һ��4��þ������״����С��������ͬ������֧�Թܺͽ�ͷ�ιܣ���Һ�¶ȿ���Ϊ298K��308K��
ͬѧ��Ϊ��̽��þ�������ᡢ���ᷴӦʱ��Ũ�Ȼ��¶ȶԷ�Ӧ���ʣ��۲�þ����ʧ��ʱ�䣩��Ӱ�죬�����������»�ѧ��Ʒ��0.2mol/L��0.40mol/L��HCl��Һ��0.2mol/L��0.40mol/L��CH3COOH��Һ��4��þ������״����С��������ͬ������֧�Թܺͽ�ͷ�ιܣ���Һ�¶ȿ���Ϊ298K��308K��