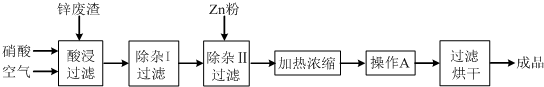
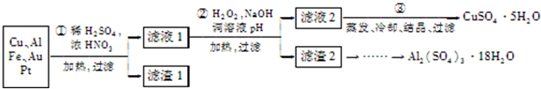
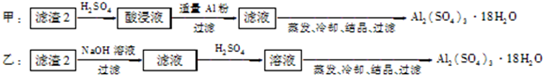
��Ŀ����
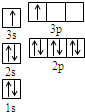 A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺
A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��Bλ��Ԫ�����ڱ���s����CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1�� Dԭ��M�ܲ�Ϊȫ����״̬����������ɶԵ��ӣ�EΪ��������δ�ɶԵ���������Ԫ�أ���ش��������⣺��1��д��E��̬ԭ�ӵĵ����Ų�ʽ
��2��ijͬѧ����������Ϣ���ƶ�B�ĺ�������Ų���ͼ��ʾ����ͬѧ�����ĵ����Ų�ͼΥ����
��3����֪A��C�γɵĻ�����X��ÿ��ԭ�ӵ�������Ϊ8�����ȶ��ṹ����X�Ļ�ѧʽΪ
��4����DԪ���γ���������Һ����ε��백ˮ�������������ӷ���ʽ��ʾ�ù��̳��ֵ�����仯��
��5����֪D��������Ӷѻ���ʽΪ�����������ܶѻ������þ�����һ�������ı߳�Ϊa cm����D������ܶ�Ϊ
���㣺λ�ýṹ���ʵ����ϵӦ��,ԭ�Ӻ�������Ų�,�����ļ���
ר�⣺
������A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ���Χ�����Ų�Ϊns2np3������̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������Ӧ��ˮ����֮���γ����������֪AΪNԪ�أ�B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��λ��Ԫ�����ڱ���s����ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s2����BΪMgԪ�أ�CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1��s�ܼ�ֻ������2�����ӣ���n=3����CΪSiԪ�أ� Dԭ��M�ܲ�Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ������ԭ�Ӻ��������Ϊ2+8+18+1=29����DΪCu��EΪ��������δ�ɶԵ���������Ԫ�أ���EΪCr���ݴ˽��
���
�⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ���֪Aԭ�ӵ�p�������3��δ�ɶԵ��ӣ���Χ�����Ų�Ϊns2np3������̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������Ӧ��ˮ����֮���γ����������֪AΪNԪ�أ�B �Ļ�̬ԭ��ռ��������״��ԭ�ӹ������������״����еĵ�����������ͬ��λ��Ԫ�����ڱ���s����ԭ�Ӻ�������Ų�ʽΪ1s22s22p63s2����BΪMgԪ�أ�CԪ��ԭ�ӵ���Χ���Ӳ��Ų�ʽΪnsn-1npn-1��s�ܼ�ֻ������2�����ӣ���n=3����CΪSiԪ�أ� Dԭ��M�ܲ�Ϊȫ����״̬���Һ����δ�ɶԵ���ֻ��һ������ԭ�Ӻ��������Ϊ2+8+18+1=29����DΪCu��EΪ��������δ�ɶԵ���������Ԫ�أ���EΪCr��
��1��CrΪ24��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d54s1�����̬ԭ����1s��2s��2p��3s��3p��3d��4s��7��������ͬ�ĵ��ӣ�
�ʴ�Ϊ��1s22s22p63s23p63d54s1��7��
��2��3p�ܼ���������3s�ܼ���Ӧ�����3s�ܼ������3p�ܼ���Υ���������ԭ�����ʴ�Ϊ���������ԭ����
��3��N��Si�γɵĻ��������������8�����ȶ��ṹ���仯ѧʽΪSi3N4��Si3N4��һ�ֳ�Ӳ���ʣ���ĥ�𡢿���ʴ����ǿ���Ʋ���Ϊԭ�Ӿ��壬
�ʴ�Ϊ��Si3N4��ԭ�Ӿ��壻
��4����CuԪ���γ���������Һ����ε��백ˮ�����������ӷ���ʽΪCu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��5��CuΪ�����������ܶѻ���������Cuԭ����Ŀ=8��
+6��
=4����������=4��
g���þ�����һ�������ı߳�Ϊa cm�������=��a cm��3=a3 cm3����Cu������ܶ�=��4��
g����a3 cm3=
g/cm3���ʴ�Ϊ��
��
��1��CrΪ24��Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d54s1�����̬ԭ����1s��2s��2p��3s��3p��3d��4s��7��������ͬ�ĵ��ӣ�
�ʴ�Ϊ��1s22s22p63s23p63d54s1��7��
��2��3p�ܼ���������3s�ܼ���Ӧ�����3s�ܼ������3p�ܼ���Υ���������ԭ�����ʴ�Ϊ���������ԭ����
��3��N��Si�γɵĻ��������������8�����ȶ��ṹ���仯ѧʽΪSi3N4��Si3N4��һ�ֳ�Ӳ���ʣ���ĥ�𡢿���ʴ����ǿ���Ʋ���Ϊԭ�Ӿ��壬
�ʴ�Ϊ��Si3N4��ԭ�Ӿ��壻
��4����CuԪ���γ���������Һ����ε��백ˮ�����������ӷ���ʽΪCu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
�ʴ�Ϊ��Cu2++2NH3?H2O=Cu��OH��2��+2NH4+��Cu��OH��2+4NH3=[Cu��NH3��4]2++2OH-��
��5��CuΪ�����������ܶѻ���������Cuԭ����Ŀ=8��
| 1 |
| 8 |
| 1 |
| 2 |
| 64 |
| NA |
| 64 |
| NA |
| 256 |
| a 3NA |
| 256 |
| a3NA |
�����������Ƕ����ʽṹ�Ŀ��飬��Ŀ�ۺ��Խϴ��漰���ӽṹ���ӻ��������������Ų��������ܡ���������ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��4���о����ռ������ʼ������ѵ㡢�״��㣬ѧϰ��ע����ػ����Ļ��ۣ��Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ����д��ȷ���ǣ�������
| A������������ϡ���ᷴӦ��Ba2++OH-+H++SO42-�TBaSO4��+H2O |
| B��ͭƬ��ϡ���ᷴӦ��Cu+NO3-+4H+�TCu2++NO��+2H2O |
| C�������ȥˮ���е�CaCO3��CaCO3+2H+�TCa2++H2O+CO2�� |
| D������������Һ�����������Һ��ϣ�2Fe2++H2O2+2H+�T2Fe3++2H2O |