��Ŀ����
���ij����ұ���������Թ�ҵ��ˮ�У�����һ������Fe3+��Cu2+��Au3+�����ӣ����ա����Ϊ������ԭ���������ͼ�еĻ������̣�Ҫ�����ó������ᡢ���ҵ�����еķ���м���Ӹù�ҵ��ˮ�л��ս𡢲���������ԭ�����������ͭ

���������������⣺
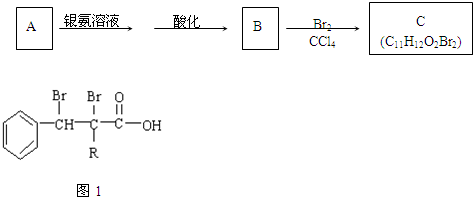
��1������ͼ�б�Ŵ���������Ӧ���ʷֱ��ǣ��� ���� ���� ���� ���� ��
��2��д������ͼ�Тٴ����ܷ��������з�Ӧ�����ӷ���ʽ��
��3��д������ͼ�Т۴�������Ӧ�Ļ�ѧ����ʽ ��

���������������⣺
��1������ͼ�б�Ŵ���������Ӧ���ʷֱ��ǣ���
��2��д������ͼ�Тٴ����ܷ��������з�Ӧ�����ӷ���ʽ��
��3��д������ͼ�Т۴�������Ӧ�Ļ�ѧ����ʽ
���㣺�����Ļ����뻷������Դ����,"����"�����뻷������
ר�⣺Ԫ�ؼ��仯����
�������ɹ�������ͼʾ֪��EΪ��Ԫ�ص����ӣ���AΪͭ����ĵ��ʣ�ͨ�����˽���Ԫ��������Ա�������ͭ��������������ˢٴ����������Ϊ��м�������ķ�ӦΪFe+2H+�TFe2++H2����2Fe3++Fe�T3Fe2+��Cu2++Fe�TCu+Fe2+��2Au3++3Fe�T2Au+3Fe2+���ڴ�����ϡ�����Գ�ȥͭ�����й�������м���������˺������������Һ�뺬Fe2+��E��Һ���ϣ��۴����ý�������������ʽ�ͭ�����뿪���ܴ����õ���ͭ��������������ת��Ϊ������ͭ�������Ӷ������ȷֽ�Ϊ����ͭ���ݴ������������ƽ���������ת��Ϊ���������������������ÿ�������ת��Ϊ���������������������ȷֽ�Ϊ���죨�����������Դ˽�����и�С�ʣ�
���
�⣺�ɹ�������ͼʾ֪��EΪ��Ԫ�ص����ӣ���AΪͭ����ĵ��ʣ�ͨ�����˽���Ԫ��������Ա�������ͭ��������������ˢٴ����������Ϊ��м�������ķ�ӦΪFe+2H+�TFe2++H2����2Fe3++Fe�T3Fe2+��Cu2++Fe�TCu+Fe2+��2Au3++3Fe�T2Au+3Fe2+���ڴ�����ϡ�����Գ�ȥͭ�����й�������м���������˺������������Һ�뺬Fe2+��E��Һ���ϣ��۴����ý�������������ʽ�ͭ�����뿪���ܴ����õ���ͭ��������������ת��Ϊ������ͭ�������Ӷ������ȷֽ�Ϊ����ͭ���ݴ������������ƽ���������ת��Ϊ���������������������ÿ�������ת��Ϊ���������������������ȷֽ�Ϊ���죨����������
��1�������Ϸ�����֪����м����ϡ�����ϡ������������ƣ����������ƣ�
�ʴ�Ϊ����м��ϡ���ϡ����������ƣ��������ƣ�
��2���ٴ����������Ϊ��м�������ķ�ӦΪFe+2H+�TFe2++H2����2Fe3++Fe�T3Fe2+��Cu2++Fe�TCu+Fe2+��2Au3++3Fe�T2Au+3Fe2+��
�ʴ�Ϊ��Fe+2H+=Fe2++H2����2Fe3++Fe=3Fe2+��Cu2++Fe=Cu+Fe2+��2Au3++3Fe=2Au+3Fe2+��
��3���۴����ý�������������ʽ�ͭ�����뿪����Ӧ�Ļ�ѧ����ʽΪ3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2O��
��1�������Ϸ�����֪����м����ϡ�����ϡ������������ƣ����������ƣ�
�ʴ�Ϊ����м��ϡ���ϡ����������ƣ��������ƣ�
��2���ٴ����������Ϊ��м�������ķ�ӦΪFe+2H+�TFe2++H2����2Fe3++Fe�T3Fe2+��Cu2++Fe�TCu+Fe2+��2Au3++3Fe�T2Au+3Fe2+��
�ʴ�Ϊ��Fe+2H+=Fe2++H2����2Fe3++Fe=3Fe2+��Cu2++Fe=Cu+Fe2+��2Au3++3Fe=2Au+3Fe2+��
��3���۴����ý�������������ʽ�ͭ�����뿪����Ӧ�Ļ�ѧ����ʽΪ3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2O��
�ʴ�Ϊ��3Cu+8HNO3��ϡ��=3Cu��NO3��2+2NO��+4H2O��
���������⿼�鳣���������仯������ۺ�Ӧ�ã���Ŀ�Ѷ��еȣ�����ע�����������������Ӧ��˳���Լ������������ױ����������ʣ�

��ϰ��ϵ�д�
�����Ŀ
����˵����ȷ���ǣ�������
| A��22.4LH2������Ϊ2g |
| B��CO2��Ħ������Ϊ44g |
| C��1molMg���������1.204��1024������ |
| D��2mol/LNa2SO4����Һ��SO42-Ϊ1.204��1024�� |
ֻ��һ��Ԫ�ص����ʣ�������
| A�������Ǵ�����Ҳ�����ǻ���� |
| B�������ǵ���Ҳ�����ǻ����� |
| C��һ���Ǵ����� |
| D��һ����һ�ֵ��� |