��Ŀ����
��80��ʱ����0.4mol�������������������2L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
��1������b c�����������=����������
��2��20sʱ��N2O4��Ũ��Ϊ mol/L��0-20s��N2O4��ƽ����Ӧ����Ϊ ��
��3��N2O4��g��?2NO2��g����ƽ�ⳣ������ʽK= ����80��ʱ�÷�Ӧ��ƽ�ⳣ��K����ֵΪ ��
��4��������������ͬʱ���÷�Ӧ��KֵԽ��������ƽ��ʱ ��
A��N2O4��ת����Խ��
B��N2O4���������Խ��
C��N2O4��NO2��Ũ��֮��Խ��
D������Ӧ���еij̶�Խ��
��5���÷�Ӧ������100s�Ժ�Ӧ�������¶Ƚ��ͣ������������ɫ��dz����÷�Ӧ�� ��Ӧ������ȡ����ȡ�����
| ʱ��/��s�� | 0 | 20 | 40 | 60 | 80 | 100 |
| c��N2O4��/��mol/L�� | 0.20 | a | 0.10 | c | d | e |
| c��NO2��/��mol/L�� | 0.00 | 0.12 | b | 0.22 | 0.24 | 0.24 |
��2��20sʱ��N2O4��Ũ��Ϊ
��3��N2O4��g��?2NO2��g����ƽ�ⳣ������ʽK=
��4��������������ͬʱ���÷�Ӧ��KֵԽ��������ƽ��ʱ
A��N2O4��ת����Խ��
B��N2O4���������Խ��
C��N2O4��NO2��Ũ��֮��Խ��
D������Ӧ���еij̶�Խ��
��5���÷�Ӧ������100s�Ժ�Ӧ�������¶Ƚ��ͣ������������ɫ��dz����÷�Ӧ��
���㣺��ѧƽ��ļ���,��ѧƽ�ⳣ���ĺ���,��ѧƽ���Ӱ������
ר�⣺
��������1�����ݱ���֪��0.12��b��0.22��c��0.10���ݴ��ж�b��c�Ĺ�ϵ��
��2�����ݶ��������������������Ĺ�ϵʽ����N2O4��Ũ�ȣ�v=
����N2O4��ƽ����Ӧ���ʣ�
��3��K=
��������80��ʱ��N2O4��NO2��ƽ��Ũ�ȴ���K�����㣻
��4��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС����Ӧ���ת����Խ��������IJ���Խ��
��5��NO2Ϊ����ɫ���壬N2O4��ɫ��
��2�����ݶ��������������������Ĺ�ϵʽ����N2O4��Ũ�ȣ�v=
| ��C |
| ��t |
��3��K=
| C2(NO2) |
| C(N2O2) |
��4��KֵԽ��������Ũ��Խ��Ӧ��Ũ��ԽС����Ӧ���ת����Խ��������IJ���Խ��
��5��NO2Ϊ����ɫ���壬N2O4��ɫ��
���
�⣺��1�����ݱ���֪��0.12��b��0.22��c��0.10������b��C��
�ʴ�Ϊ��b��C��
��2��20sʱ��������������Ũ��Ϊa��
N2O4?2NO2
1mol/L 2mol/L
��0.20-a��mol/L 0.12mol/L
a=0.14mol/L����Ӧ��N2O4Ũ��Ϊ��0.2-0.14��mol/L=0.06mol/L��
v��N2O4��=
=
=0.003mol/L��s��
�ʴ�Ϊ��0.14��0.003mol/��L��s����
��3��K=K=
����80sʱ����Ӧ��ƽ�⣬���ڴ�ʱNO2��ƽ��Ũ��Ϊ0.24mol/L�����ݷ�Ӧ��֪��
N2O4 ?2NO2
��ʼC��0.2mol/L 0
�ı�C��0.12mol/L 0.24mol/L
ƽ��C��0.08mol/L 0.24mol/L
N2O4��ƽ��Ũ��Ϊ0.08mol/L����NO2��ƽ��Ũ��Ϊ0.24mol/L����80��ʱƽ�ⳣ��K=
=
=0.72
�ʴ�Ϊ��K=
��0.72��
��4�����ݻ�ѧƽ�ⳣ��֪��KԽ���������Ũ��Խ��Ӧ���Ũ��ԽС��N2O4��ת���ʴ�N2O4���������ԽС��N2O4��NO2��Ũ��֮��ԽС������Ӧ���еij̶�Խ��ѡAD��
�ʴ�Ϊ��AD��
��5��NO2Ϊ����ɫ���壬N2O4��ɫ���ʷ�Ӧ�������¶Ƚ��ͣ������������ɫ��dz��˵��ƽ�����ƣ�������Ӧ�������ȣ��ʴ�Ϊ�����ȣ�
�ʴ�Ϊ��b��C��
��2��20sʱ��������������Ũ��Ϊa��
N2O4?2NO2
1mol/L 2mol/L
��0.20-a��mol/L 0.12mol/L
a=0.14mol/L����Ӧ��N2O4Ũ��Ϊ��0.2-0.14��mol/L=0.06mol/L��
v��N2O4��=
| ��C |
| ��t |
| 0.06mol/L |
| 20s |
�ʴ�Ϊ��0.14��0.003mol/��L��s����
��3��K=K=
| C2(NO2) |
| C(N2O2) |
N2O4 ?2NO2
��ʼC��0.2mol/L 0
�ı�C��0.12mol/L 0.24mol/L
ƽ��C��0.08mol/L 0.24mol/L
N2O4��ƽ��Ũ��Ϊ0.08mol/L����NO2��ƽ��Ũ��Ϊ0.24mol/L����80��ʱƽ�ⳣ��K=
| C2(NO2) |
| C(N2O2) |
| (0.24)2 |
| 0.08 |
�ʴ�Ϊ��K=
| C2(NO2) |
| C(N2O2) |
��4�����ݻ�ѧƽ�ⳣ��֪��KԽ���������Ũ��Խ��Ӧ���Ũ��ԽС��N2O4��ת���ʴ�N2O4���������ԽС��N2O4��NO2��Ũ��֮��ԽС������Ӧ���еij̶�Խ��ѡAD��
�ʴ�Ϊ��AD��
��5��NO2Ϊ����ɫ���壬N2O4��ɫ���ʷ�Ӧ�������¶Ƚ��ͣ������������ɫ��dz��˵��ƽ�����ƣ�������Ӧ�������ȣ��ʴ�Ϊ�����ȣ�
���������⿼���˻�ѧ��Ӧ���ʵļ��㡢��ѧƽ�ⳣ���ĺ����֪ʶ�㣬ע�⻯ѧƽ�ⳣ��ֻ���¶��йأ������ʵ�Ũ���أ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
±���� C5H11Cl��C5H12O���ڴ���Ľṹ���ж����֣�������
| A��5��3 | B��3��5 |
| C��10��8 | D��8��8 |
��ȥij��Һ���ܽ���������ʣ����������в���ȷ���ǣ������ڵ�����Ϊ���ʣ���������
| A��FeCl2��Һ��FeCl3�����ӹ�����ԭ���ۣ����� |
| B��KNO3��Һ��AgNO3�����ӹ���KCl ��Һ������ |
| C��NaCl��Һ��I2������CCl4����Һ |
| D��KNO3��Һ��NaCl��������������Ũ��Һ���¡����� |
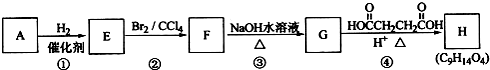

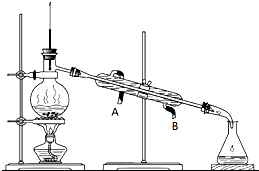 ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����
ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����