��Ŀ����
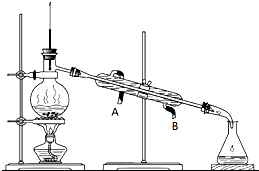 ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����
ij������Һ���ⶨ��֪��Ҫ�����Ҵ������л��б�ͪ������������������������и����ʵ�����| ���� | ��ͪ | �������� | �Ҵ� | ���� |
| �е�/�� | 56.2 | 77.06 | 78.5 | 117.9 |
������������
�����в�������Ҵ������
�����Һ�м����ռ���Һ��������Һ��pH=10��
�ڽ����Һ�����������л������ȣ�
���ռ��¶���70�桫85��ʱ������
���ų��������еIJ�Һ����ȴ�������м�Ũ���ᣨ��������Ȼ���ٷ��������������н�������������
����װ����ͼ����ش��������⣺
��1�������ռ�ʹ��Һ��pH=10��Ŀ����
��2������������ȴˮ������
��3���ڲ�����м������Ũ�����Ŀ���ǣ��û�ѧ����ʽ��ʾ��
��4����70�桫85��ʱ��������Ҫ�ɷ���
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺
�������������ʵķе㣬���Ǽ���������������Һ��ʾ���ԣ�����������ת��Ϊ�����ƺ��Ҵ���������ת��Ϊ�����ƣ�Ȼ������¶ȣ�����Ҫ����������������õ��Ļ��Һ�м���Ũ���ᣬ��������ת��Ϊ���ᣬ�����¶ȣ��ɽ������������ݴ˻شɣ�
���
�⣺��1�������ռ�ʹ��Һ��pH=10��Ŀ���ǽ�����ת��Ϊ�����ƣ�ʹ����������ת��Ϊ�����ƺ��Ҵ���
�ʴ�Ϊ��������ת��Ϊ�����ƣ�ʹ����������ת��Ϊ�����ƺ��Ҵ���
��2������������ȴˮ�½��ϳ���������B����ƿ�������Ӵ�Ƭ�������Ƿ�ֹ���У��ʴ�Ϊ��B����ֹ���У�
��3���ڲ�����м������Ũ�����Ŀ���Ǻʹ����Ʒ�Ӧ���ɴ��ᣬ����ʽΪ2CH3COONa+H2SO4��Na2SO4+2CH3COOH���ʴ�Ϊ��2CH3COONa+H2SO4��Na2SO4+2CH3COOH��
��4������ͼ�������ʷе������֪����70�桫85��ʱ��������Ҫ�ɷ����Ҵ��������������¶ȿ�����85�桫125��һ��ʱ���������������Һ�����ʵ���Ҫ�ɷ����ݷ�Ӧ2CH3COONa+H2SO4-��Na2SO4+2CH3COOH ��֪���õ���Һ�����ʵ���Ҫ�ɷ��������ƣ��ʴ�Ϊ���Ҵ��� Na2SO4��
�ʴ�Ϊ��������ת��Ϊ�����ƣ�ʹ����������ת��Ϊ�����ƺ��Ҵ���
��2������������ȴˮ�½��ϳ���������B����ƿ�������Ӵ�Ƭ�������Ƿ�ֹ���У��ʴ�Ϊ��B����ֹ���У�
��3���ڲ�����м������Ũ�����Ŀ���Ǻʹ����Ʒ�Ӧ���ɴ��ᣬ����ʽΪ2CH3COONa+H2SO4��Na2SO4+2CH3COOH���ʴ�Ϊ��2CH3COONa+H2SO4��Na2SO4+2CH3COOH��
��4������ͼ�������ʷе������֪����70�桫85��ʱ��������Ҫ�ɷ����Ҵ��������������¶ȿ�����85�桫125��һ��ʱ���������������Һ�����ʵ���Ҫ�ɷ����ݷ�Ӧ2CH3COONa+H2SO4-��Na2SO4+2CH3COOH ��֪���õ���Һ�����ʵ���Ҫ�ɷ��������ƣ��ʴ�Ϊ���Ҵ��� Na2SO4��
�����������漰�л���������Լ����ʵķ��뷽�������������ʵķе�ʵ�����ʵķ��룬���ջ����ǹؼ����ѶȲ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
ͬ��ͬѹ�£���3֧�����ͬ���Թ��зֱ�����������ϵ�2�����壬�����ǣ���NO��NO2����NO2��O2����NH3��N2���ֽ�3֧�Թܾ�������ˮ���У���ַ�Ӧ���Թ������ʵ����ʵ���Ũ�ȷֱ�Ϊc1��c2��c3����������ȫ���Թ��У��������й�ϵ��ȷ���ǣ�������
| A��c1��c2��c3 |
| B��c1��c3��c2 |
| C��c1=c3��c2 |
| D��c1=c2��c3 |
�������ʣ����侧����۵��ɸߵ���˳�����е��ǣ�������
| A��H2O��H2S��H2Se��H2Te |
| B��C��CH3��4��CH��CH3��2CH2CH3��CH3��CH2��3CH3 |
| C��SiO2��NaCl��CCl4��SO2 |
| D��F2��Cl2��Br2��I2 |


 ���������飨
���������飨 ��
�� ��
��
 ��Ȳ��һ����Ҫ���л�����ԭ�ϣ�����ȲΪԭ���ڲ�ͬ�ķ�Ӧ�����¿���ת�������»����������и��⣺
��Ȳ��һ����Ҫ���л�����ԭ�ϣ�����ȲΪԭ���ڲ�ͬ�ķ�Ӧ�����¿���ת�������»����������и��⣺