��Ŀ����
ij�����о�С�飬�ú��н϶����ʵ�ͭ�ۣ�ͨ����ͬ�Ļ�ѧ��Ӧ��ȡ����������Ƶ�ʵ�����Ϊ����������ͭ�л����н϶��ͭ����

���������գ�
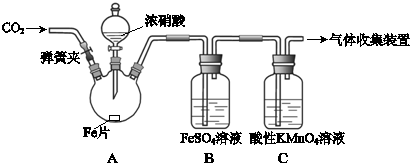
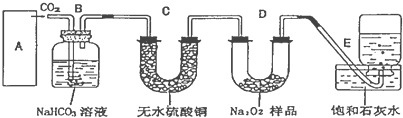
��1��ͭ�к��д������л���ɲ������յķ�����ȥ�л������ʱ�����żܡ�����ǯ���������⣬ʵ����������������� ��
��2��ͨ��;����ʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ� ����ȴ�ᾧ�� ����Ȼ���
��3���ɴ�������ͭͨ������;����ȡ��������;�������;������ŵ��ǣ� �� ��
��4�����ڲⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��ʵ������У������������ٽ��� �Σ�
��5�����ⶨ���xֵƫ�ߣ����ܵ�ԭ����
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯��

���������գ�
��1��ͭ�к��д������л���ɲ������յķ�����ȥ�л������ʱ�����żܡ�����ǯ���������⣬ʵ�����������������
��2��ͨ��;����ʵ���ô�������ͭ��ȡ������������е�ʵ��������裺���ܡ�����ͨ���������ˡ�
��3���ɴ�������ͭͨ������;����ȡ��������;�������;������ŵ��ǣ�
��4�����ڲⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��ʵ������У������������ٽ���
��5�����ⶨ���xֵƫ�ߣ����ܵ�ԭ����
a�������¶ȹ��� b����������Ŀ����ϴ�
c�����Ⱥ���ڿ�������ȴ d���������岿�ַ绯��
���㣺�Ʊ�ʵ�鷽�������,���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��
ר�⣺ʵ�������
��������1�����ݸ�������ȵIJ������������ж���Ҫ��������Ȼ��д����ȱ�ٵ��������ƣ�
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壻
��3��;������Ũ����μӷ�Ӧ�����Ľ϶����ᣬ��������Ⱦ�Զ����������壻
��4��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ����仯��������4�Σ�
��5��a���¶ȹ��ߣ��ᵼ������ͭ�ֽ⣬�����仯�ϴ�
b����������Ŀ����ϴᵼ�¾�����ȷֽⲻ��ȫ�������仯ƫС��
c���ڿ�������ȴ�������տ����е�ˮ�����γɾ��壻
d���������岿�ַ绯����ʹˮ�ĺ�����С�����ƫС��
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壻
��3��;������Ũ����μӷ�Ӧ�����Ľ϶����ᣬ��������Ⱦ�Զ����������壻
��4��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ����仯��������4�Σ�
��5��a���¶ȹ��ߣ��ᵼ������ͭ�ֽ⣬�����仯�ϴ�
b����������Ŀ����ϴᵼ�¾�����ȷֽⲻ��ȫ�������仯ƫС��
c���ڿ�������ȴ�������տ����е�ˮ�����γɾ��壻
d���������岿�ַ绯����ʹˮ�ĺ�����С�����ƫС��
���
�⣺��1����Ʒ��Ҫ���ڴ������С��þƾ��Ƽ��ȡ�����ʱ���������������ż��ϵ��������ϼ��ȡ����ȹ�������Ҫ�ò��������衢�ƶ���������Ҫʹ������ǯ�����Ⱥ����ʯ��������ȴ����������ʱ�����żܡ�����ǯ���������⣬ʵ����������������У����������ƾ��ơ���������
�ʴ�Ϊ�����������ƾ��ơ���������
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壬
�ʴ�Ϊ�����������ˣ�
��3���ȽϷ�Ӧ������;����;������Ũ����μӷ�Ӧ�����Ľ϶����ᣬ��������Ⱦ�Զ����������壬������;�������;������ŵ��ǣ�������������;�� II���������٣�;��II���������Ⱦ����������
�ʴ�Ϊ��������������;�� II���������٣�;��II���������Ⱦ���������壻
��4���ⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ������������Χ�ڼ�����ֵ�Ƿ�������0.1g��������4�Σ�
�ʴ�Ϊ��4��
��5��a�������¶ȹ��ߣ��ᵼ������ͭ�ֽ⣬�����仯�ϴ��½��ƫ��a��ȷ��
b����������Ŀ����ϴᵼ�¾�����ȷֽⲻ��ȫ�������仯ƫС�����ƫС����b����
c�����Ⱥ���ڿ�������ȴ�������տ����е�ˮ�����γɾ��壬���ƫС����c����
d���������岿�ַ绯����ʹˮ�ĺ�����С�����ƫС����d����
�ʴ�Ϊ��a��
�ʴ�Ϊ�����������ƾ��ơ���������
��2��������ͭ��Һ�Ƶ�����ͭ���壬�����˺������������ͭ��Һ����ȴ�ᾧ���ˡ������ɵô���������ͭ���壬
�ʴ�Ϊ�����������ˣ�
��3���ȽϷ�Ӧ������;����;������Ũ����μӷ�Ӧ�����Ľ϶����ᣬ��������Ⱦ�Զ����������壬������;�������;������ŵ��ǣ�������������;�� II���������٣�;��II���������Ⱦ����������
�ʴ�Ϊ��������������;�� II���������٣�;��II���������Ⱦ���������壻
��4���ⶨ���õ�����CuSO4?xH2O���нᾧˮxֵ��Ӧ���������������������;�������������Ⱥ����������������Ⱥ��ٳ���һ���������������ж������Ƿ������������Χ�ڼ�����ֵ�Ƿ�������0.1g��������4�Σ�
�ʴ�Ϊ��4��
��5��a�������¶ȹ��ߣ��ᵼ������ͭ�ֽ⣬�����仯�ϴ��½��ƫ��a��ȷ��
b����������Ŀ����ϴᵼ�¾�����ȷֽⲻ��ȫ�������仯ƫС�����ƫС����b����
c�����Ⱥ���ڿ�������ȴ�������տ����е�ˮ�����γɾ��壬���ƫС����c����
d���������岿�ַ绯����ʹˮ�ĺ�����С�����ƫС����d����
�ʴ�Ϊ��a��
����������ͨ���������Ʊ����̣������������Ʊ���������ƣ��漰ͭ���仯���������Ӧ�á���ѧʵ���������������������֪ʶ����Ŀ�Ѷ��еȣ�ע�����������Ʊ������ķ�������ȷ�Ʊ�ԭ������ȷ�IJ�������Ϊ���ؼ���

��ϰ��ϵ�д�
�����Ŀ
������ʵ��������������ԭ�����͵��ǣ�������
| A��������ˮ��Һ�м����������S2-������ |
| B��������������ڰ���������Ӧ |
| C����ѹ�����ںϳɰ���Ӧ |
| D����������ˮ�м���̼��������ڴ�����Ũ������ |
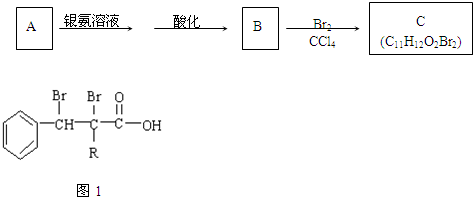

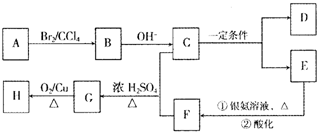
 ��R��R�����������Ҳ��������ԭ�ӣ�
��R��R�����������Ҳ��������ԭ�ӣ�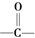 ���칹�干�и�
���칹�干�и�