��Ŀ����
������ԴΣ���ļӾ磬�ҹ�Ҳ�ڴ����ƹ���һ���Ҵ�ȼ�ϣ��������ҹ��������������֮һ���Ҵ����ԴӸ����������ӹ�������

��1��д��������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ�� ��
��2���Ҵ�������ȼ���⣬�����������ϳ������л�����������Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵ��B���ճ������г�������ʳƷ��װ����������������������⣬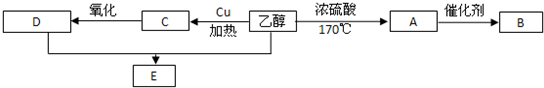
��ش�������⣺
д����A��B�Ļ�ѧ����ʽ�� ��
��д���Ҵ���A�Ļ�ѧ����ʽ�� ��
��д���Ҵ���C�Ļ�ѧ����ʽ ��
��д������E�Ļ�ѧ����ʽ ��
��3����������A��B��C��D��E�����ǡ������ǡ��Ҵ��������ܺ�����������ͭ�ڼ��������·�Ӧ����ש��ɫ�������� ����д���ƣ�

��1��д��������ת��Ϊ�Ҵ��Ļ�ѧ����ʽ��
��2���Ҵ�������ȼ���⣬�����������ϳ������л�����������Ҵ�Ϊ��ʼԭ�ϵ�ת����ϵ��B���ճ������г�������ʳƷ��װ����������������������⣬

��ش�������⣺
д����A��B�Ļ�ѧ����ʽ��
��д���Ҵ���A�Ļ�ѧ����ʽ��
��д���Ҵ���C�Ļ�ѧ����ʽ
��д������E�Ļ�ѧ����ʽ
��3����������A��B��C��D��E�����ǡ������ǡ��Ҵ��������ܺ�����������ͭ�ڼ��������·�Ӧ����ש��ɫ��������
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
��������1���������ھƻ�ø�����µõ��ƾ��������̼��
��2���Ҵ���������������DΪCH3CHO��D��һ������������Ӧ����DΪCH3COOH���Ҵ���D����������Ӧ����EΪCH3COOCH2CH3���Ҵ�������ȥ��Ӧ����AΪCH2=CH2��B���ճ������г�������ʳƷ��װ��������ϩ�����Ӿ۷�Ӧ����BΪ ��
��
��3����ȩ����������������������ͭ�ڼ��������·�Ӧ����ש��ɫ������
��2���Ҵ���������������DΪCH3CHO��D��һ������������Ӧ����DΪCH3COOH���Ҵ���D����������Ӧ����EΪCH3COOCH2CH3���Ҵ�������ȥ��Ӧ����AΪCH2=CH2��B���ճ������г�������ʳƷ��װ��������ϩ�����Ӿ۷�Ӧ����BΪ
 ��
����3����ȩ����������������������ͭ�ڼ��������·�Ӧ����ש��ɫ������
���
�⣺��1���������ھƻ�ø�����µõ��ƾ��������̼����Ӧ����ʽΪ��C6H12O6
2CH3CH2OH+2CO2�����ʴ�Ϊ��C6H12O6
2CH3CH2OH+2CO2����
��2���Ҵ���������������DΪCH3CHO��D��һ������������Ӧ����DΪCH3COOH���Ҵ���D����������Ӧ����EΪCH3COOCH2CH3���Ҵ�������ȥ��Ӧ����AΪCH2=CH2��B���ճ������г�������ʳƷ��װ��������ϩ�����Ӿ۷�Ӧ����BΪ ��
��
��A��B�Ļ�ѧ����ʽΪ��n CH2=CH2
 ��
��
���Ҵ���A�Ļ�ѧ����ʽ��CH3CH2OH
CH2=CH2��+H2O��
���Ҵ���C�Ļ�ѧ����ʽ��2CH3CH2OH+O2
2CH3CHO+H2O��
������E�Ļ�ѧ����ʽ��CH3COOH+C2H5OH
CH3COOCH2CH3+2H2O��
�ʴ�Ϊ��n CH2=CH2
 ��CH3CH2OH
��CH3CH2OH
CH2=CH2��+H2O��
2CH3CH2OH+O2
2CH3CHO+2H2O��CH3COOH+C2H5OH
CH3COOCH2CH3+H2O��
��3����ȩ�������Ǻ���ȩ������������������ͭ�ڼ��������·�Ӧ����ש��ɫ�������ʴ�Ϊ����ȩ�������ǣ�
| �ƻ�ø |
| �ƻ�ø |
��2���Ҵ���������������DΪCH3CHO��D��һ������������Ӧ����DΪCH3COOH���Ҵ���D����������Ӧ����EΪCH3COOCH2CH3���Ҵ�������ȥ��Ӧ����AΪCH2=CH2��B���ճ������г�������ʳƷ��װ��������ϩ�����Ӿ۷�Ӧ����BΪ
 ��
����A��B�Ļ�ѧ����ʽΪ��n CH2=CH2
| ���� |
 ��
�����Ҵ���A�Ļ�ѧ����ʽ��CH3CH2OH
| Ũ���� |
| 170�� |
���Ҵ���C�Ļ�ѧ����ʽ��2CH3CH2OH+O2
| Cu |
| �� |
������E�Ļ�ѧ����ʽ��CH3COOH+C2H5OH
| Ũ���� |
| �� |
�ʴ�Ϊ��n CH2=CH2
| ���� |
 ��CH3CH2OH
��CH3CH2OH| Ũ���� |
| 170�� |
2CH3CH2OH+O2
| Cu |
| �� |
| Ũ���� |
| �� |
��3����ȩ�������Ǻ���ȩ������������������ͭ�ڼ��������·�Ӧ����ש��ɫ�������ʴ�Ϊ����ȩ�������ǣ�
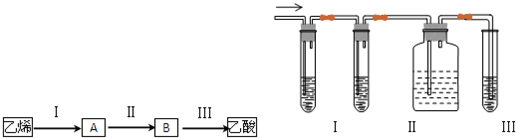
���������⿼���л����ƶϣ�ˮ�ⴼ��ȩ�����ᡢϩ�����ǵ�������ת�����ѶȲ����ضԻ���֪ʶ�Ĺ��̣�

��ϰ��ϵ�д�
�����Ŀ

 ��
��

 ��״�ṹ��ͬ���칹�������
��״�ṹ��ͬ���칹�������