��Ŀ����
4��H2O2���ȶ����ֽ⣬Fe3+��Cu2+�ȶ���ֽ�������ã�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С��ͬѧ�ֱ������ͼ1ͼ2����ʵ��װ�ã�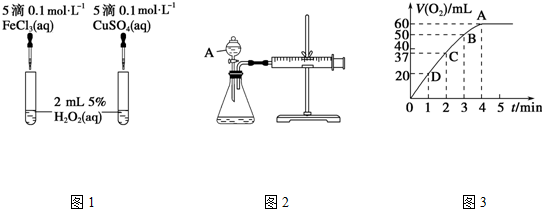
��1��������ͼ1ʵ�飬��ͨ���۲�������ݵĿ������Ӷ����ԱȽϵó����ۣ���ͬѧ�����FeCl3��ΪFe2��SO4��3��Ϊ�����������������������Ӳ�ͬ��ʵ��ĸ��ţ�д��H2O2�ڶ������̴������·�����Ӧ�Ļ�ѧ����ʽ��2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2����������ͼ2ʵ��ɽ��ж���������ͼ������A������Ϊ��Һ©����ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�ʵ���л���Ҫ�����������Dz���40mL���������ʱ�䣮
��2����0.1mol MnO2��ĩ����50mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ3��ʾ�����ͷ�Ӧ���ʱ仯��ԭ�����ŷ�Ӧ�Ľ��У�Ũ�ȼ�С����Ӧ���ʼ�����H2O2��ʼ���ʵ���Ũ��Ϊ0.11 mol/L��������λ��Ч���֣���
��3��Ϊ�˼���ͬѧ�Ƕ�Ӱ�췴Ӧ�������ص���ʶ����ʦ��ͬѧ�������ʵ�飺������ʵ��װ�õ���ƿ�ڼ���6.5gп����ͨ����Һ©������40mL 2.5mol•L-1�����ᣬ10sʱ�ռ�������H2�����Ϊ44.8mL����״�������������ʾ10s�ڸ÷�Ӧ������Ϊ0.25 mol•L-1•s-1�����跴Ӧǰ����Һ��������䣩��
���� ��1���ȽϷ�Ӧ���ʵĴ�С��ͨ���������ݵĿ������жϣ��ȽϽ��������ӵĴ�Ч��Ҫ�ų������ӵĸ��ţ�A���������Ƿ�Һ©��������װ�������Եķ����ǣ��رշ�Һ©���Ļ�������ע����������������һ�Σ���һ������Ƿ�ص�ԭλ���÷�Ӧ��ͨ����Ӧ���ʷ����ģ����Ը���v=$\frac{��c}{��t}$������
��2�����ŷ�Ӧ�Ľ��У���Һ��Ũ�����ͣ���Ӧ������С������ͼ����Կ���H2O2��ȫ��Ӧ�ų�60 mL O2������2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2�����м��㣻
��3������2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2����v=$\frac{��c}{��t}$���м��㣮
��� �⣺��1���÷�Ӧ�в������壬�ɸ����������ݵĿ����жϣ��Ȼ���������ͭ���������Ӷ���ͬ�����ж��������������û��������������ã������ƺ�����ͭ��������ͬ���������������Ӳ�ͬ��ʵ��ĸ��ţ��ڶ������������������£�˫��ˮ�ֽ�����ˮ����������Ӧ����ʽΪ��2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2����A���������Ƿ�Һ©��������װ�������Եķ����ǣ��رշ�Һ©���Ļ�������ע����������������һ�Σ���һ������Ƿ�ص�ԭλ��������ָ�ԭλ����֤�����������ã����ã�����v=$\frac{��c}{��t}$֪������Ҫ�ⶨ����40mL���������ʱ�䣬
�ʴ�Ϊ���������ݵĿ��������������Ӳ�ͬ��ʵ��ĸ��ţ�2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2������Һ©��������40mL���������ʱ�䣻
��2���ڶ������������������£�˫��ˮ�ֽ�����ˮ����������Ӧ����ʽΪ��2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2����Ũ��Խ��Ӧ����Խ��֮ԽС�����ŷ�Ӧ���У���Ӧ���Ũ����С����������С������ͼ����Կ���H2O2��ȫ��Ӧ�ų�60 mL O2��H2O2�ķֽⷴӦΪ2H2O2$\frac{\underline{\;����\;}}{\;}$2H2O+O2������n��H2O2��=$\frac{0.06}{22.4}$��2��0.00536 mol��
c��H2O2��=$\frac{0.00536}{0.05}$��0.11 mol/L���ʴ�Ϊ�����ŷ�Ӧ�Ľ��У�Ũ�ȼ�С����Ӧ���ʼ�����0.11 mol/L��
��3��Zn+2H+=Zn2++H2
65g 1mol 1mol 22.4L
6.5g 0.1mol 0.1mol 0.0448L
�������ʾ10s�ڸ÷�Ӧ������Ϊv=$\frac{\frac{0.1}{0.04}}{10}$=0.25mol•L-1•s-1���ʴ�Ϊ��0.25��
���� ���⿼��ʵ�鷽����ƣ�����v=$\frac{��c}{��t}$�����ʵ�飬ͨ���õ�����������ʱ��Ĺ�ϵȷ��Ӱ�췴Ӧ���ʵ����أ��Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��3���ڢ�A�� | B�� | ��3���ڢ�B�� | C�� | ��3���ڢ�B�� | D�� | ��3���ڢ�A�� |
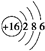
 �����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ����ͼ��ʾ��
�����������ڽ���Na������S�Ͷ����ƣ�Na2SX���ֱ���Ϊ�����缫�ķ�Ӧ������Al2O3�մɣ��ɴ���Na+��Ϊ����ʣ��䷴Ӧԭ����ͼ��ʾ��Na2SX$?_{�ŵ�}^{���}$2Na+XS ��3��X��5��
| ���� | Na | S | Al2O3 |
| �۵�/�� | 97.8 | 115 | 2050 |
| �е�/�� | 892 | 444.6 | 2980 |
A��100������ B��100�桫300��
C��300�桫350��D��350�桫2050��
��5�����������أ�����˵����ȷ����AD������ĸ��ţ���
A���ŵ�ʱ���缫AΪ����
B���ŵ�ʱ��Na+���ƶ�����Ϊ��B��A
C�����ʱ���缫AӦ���ӵ�Դ������
D�����ʱ�缫B�ĵ缫��ӦʽΪSX2--2e-=xS
��6��25��ʱ��������������Ϊ��Դ���500mL 0.2mol•L-1 NaCl��Һ������Һ��pH��Ϊl3ʱ�����Ե��ǰ����Һ������仯������·��ͨ���ĵ��ӵ����ʵ���Ϊ
0.05mol�������������ķ�Ӧ���������Ϊ2.3 g����������ǰ�����������ķ�Ӧ��������ȣ�
| X | |
| Y | Z |
��2��Yԭ�ӵĽṹʾ��ͼΪ
 ��U2X�ĵ���ʽ
��U2X�ĵ���ʽ
��3��YX2��U2Y��Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2�T3S��+2H2O��������������SO2����������Ԫ����S��
| ѡ�� | A | B | C | D |
| �������� | �⻯�� | �ɱ� | ʯī | �� |
| ������ ������ | ���������� | ���� | ԭ�� | ���� |
| ���Ӽ� ������ | ���Ӽ� | ���Ӽ� ������ | ���ۼ� | ���Ӽ� ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ͬ���칹�� | B�� | ͬ�������� | C�� | ͬλ�� | D�� | ͬϵ�� |
| A�� | �Ҵ������顢������ | B�� | �������Ȼ�̼����ϩ | ||
| C�� | �����ױ��������� | D�� | �״����������������� |
��1��FeԪ�������ڱ��е�λ���ǵ������ڵڢ����壬��֤��������д��ڼ�Ԫ�صķ���������ɫ��Ӧʵ�飬����ɫ�ܲ����۲�������ɫ����ɫ��
��2�����������������������ɼס��Һ�ˮ������ɣ��ס���ת����ϵ��ͼ����ת��ʱ�������仯��ͼ������֪A���γɻ�������������Ԫ�أ�B�ǵؿ��к�������Ԫ�أ�X��Y���ס����Ǻ�A��B��Ԫ�صĵ��ʻ�������ҵĵ���ʽΪ
 ��д����ӦX+Y�����Ȼ�ѧ����ʽ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��д����ӦX+Y�����Ȼ�ѧ����ʽ��C��s��+$\frac{1}{2}$O2��g��=CO��g����H=-110.6KJ/mol��
��3����С��ͬѧ��֪��������У���Ԫ�ز���������ʽ���ڣ�����Ҳֻ��K2AB3��Ϊ�˽�һ��ȷ������M�ķֽⷴӦ����ʽ��С��ͬѧ�Թ��������ж���������
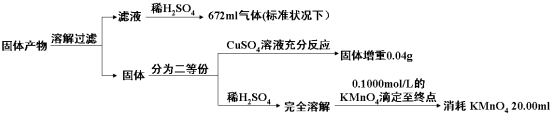
�ж�KMnO4���仹ԭ����Ϊ��ɫMn2+��Һ����Һ��������Ӧ����ζ��յ������Ϊ���������һ�����Ը��������Һʱ��������ɫ�仯Ϊ�Ϻ�ɫ��������ڲ��仯��������ʵ�����ݵķ�����֪�����Ⱥ����ù�������г�K2AB3�����ʲô���ʣ�����֮������ʵ���֮��Ϊ���٣������Ľ���������У����Բ�������ո�
| ��������еijɷ� | K2AB3 | �� | ||
| ���ʵ���֮�� | ||||
��5����֪KHA2B4��Һ�����ԣ���10mL0.01mol•L-1��H2A2O4��Һ�μ�0.01mol•L-1KOH��ҺV��mL��������������ȷ���Ǣ٢ڢۣ�
�ٵ�V��10mLʱ����Ӧ�����ӷ���ʽΪH2A2B4+OH-=HA2B4++H2O
�ڵ�V=10mLʱ����Һ��C��HA2B4+����C��H+����C��A2B42-����C��H2A2B4��
�۵�V=amLʱ����Һ������Ũ�ȵĹ�ϵΪc��K+��=2c��A2B42-��+c��HA2B4-��
��V=bmLʱ����Һ������Ũ�ȵĹ�ϵΪ��c��K+��=c��A2B42-��+c��HA2B4-��+c��H2A2B4������a��b��