��Ŀ����
16��ʵ�����Ʊ��������ķ�Ӧԭ����ʵ��װ�����£�
���ڵ���Ҫ����Ӧ�У����¶��Ըߵ�����»����ɼ�����������й����������
| ���� | �۵�/�� | �е�/�� | �ܶ�/g•cm-3 | �ܽ��� |
| �� | 5.5 | 80 | 0.88 | ����ˮ |
| ������ | 5.7 | 210.9 | 1.205 | ������ˮ |
| ��������� | 89 | 301 | 1.57 | ����ˮ |
| Ũ���� | 83 | 1.4 | ������ˮ | |
| Ũ���� | 338 | 1.84 | ������ˮ |
ȡ100mL�ձ�����20mLŨ������18mLŨ�������ƻ��Һ���������С�ļ���B�У���17.73mL��15.6g��������A�У��������µı�����μ�����ᣬ�ߵα߽��裬��Ͼ��ȣ���50��60���·�����Ӧ��ֱ����Ӧ������
����ӦҺ��ȴ�����º����Һ©���У�����������ˮ��5% NaOH��Һ��ˮϴ�ӣ��ֳ��IJ��������ˮCaCl2����������Ƭ�̣���ȥCaCl2�������������ռ�205��210����֣��õ����������18.45g���ش��������⣺

��1��װ��B�������Ƿ�Һ©����װ��C������������������
��2�����ƻ��Һʱ�����ܣ���ܡ����ܡ�����Ũ������뵽Ũ�����У�˵�����ɣ��������Ž���
��3��Ϊ��ʹ��Ӧ��50��60���½��У����õķ�����ˮԡ���ȣ�
��4����ϴ�Ӳ����У��ڶ���ˮϴ��������ϴȥ������NaOH�����ɵ��Σ�
��5����ʵ�����õ���������������75%��������λ��Ч���֣���
���� ��1��װ��BΪ��Һ©����װ��CΪ���������ܣ��������������ã�
��2�����ƻ���Ӧ��ֹҺ��Ž���
��3����Ӧ��50�桫60���½��У�����ˮԡ���ȿ��ƣ�
��4������������ϴȥ�ܽ������ᣬˮϴ��ȥδ��Ӧ��NaOH�����ɵ��Σ�
��5�����ݱ����������������������۲���������=��ʵ�ʲ��������۲�������100%��
��� �⣺��1���������ṹ������֪��װ��BΪ��Һ©����װ��CΪ���������ܣ�����Ũ���ᶼ�Իӷ���C�������������ã����ԭ�������ʣ�
�ʴ�Ϊ����Һ©��������������
��2��Ũ������Ũ�����ϻ�ų��������ȣ��罫Ũ�������Ũ�����У�������ܶ�С��Ũ���ᣬ����Ϊ����Һ��Ž���
�ʴ�Ϊ�����ܣ��������Ž���
��3����Ӧ��50�桫60���½��У�����ˮ�ķе㣬��������ˮԡ���ȿ��ƣ����Ⱦ��ȣ�
�ʴ�Ϊ��ˮԡ���ȣ�
��4������ˮϴ��ȥŨ���ᡢ���ᣬ�����������Ƴ�ȥ�ܽ�������ᣬ���ˮϴ��ȥδ��Ӧ��NaOH�����ɵ��Σ�
�ʴ�Ϊ��ϴȥ������NaOH�����ɵ��Σ�
��5������ȫ��Ӧ���������������۲���Ϊ15.6g��$\frac{123}{78}$�����������IJ���Ϊ[18.45g�£�15.6g��$\frac{123}{78}$��]��100%=75%��
�ʴ�Ϊ��75%��
���� ���⿼���л����Ʊ�ʵ�飬�漰��װ�ü������ķ������ۡ����ʵķ����ᴿ�����ʼ���ȣ��Ѷ��еȣ�ע������淶�Լ��������á�����ȫ��ʶ��

| A�� | ���� | B�� | ʯ�� | C�� | �ȷ� | D�� | �ѽ��� |
| A�� | ͨ�����SO2��������ܴ�������H+��Fe2+��I?��SO42? | |
| B�� | ʹʯ�������Һ�п��ܴ�������Na+��Mg2+��NO3?��C17H35COO? | |
| C�� | ǿ������Һ�п��ܴ�������Na+��K+��Cl-��HCO3- | |
| D�� | ����Al ���Էų�H2����Һ�п��ܴ�������Fe3+��K+��Cl?��NO3- |
| A�� | pH��ȵĢ�NaHCO3����Na2CO3����NaOH��Һ�����ʵ���Ũ�ȴ�С���ۣ��ڣ��� | |
| B�� | Ũ�Ⱦ�Ϊ0.1mol/L�Ģ٣�NH4��2CO3���ڣ�NH4��2SO4���ۣ�NH4��2Fe��SO4��2��Һ�У�c��NH4+���Ĵ�С˳��Ϊ���٣��ڣ��� | |
| C�� | ��NH4Cl��Һ�м���ϡHNO3��������NH4+ˮ�� | |
| D�� | ��CH3COONa��Һ�м�������ᣬ�ܿ���CH3COO-ˮ�� |
| A�� | ������ӦʽΪMg-2e-�TMg2+ | |
| B�� | ������ӦʽΪAg+e-�TAg | |
| C�� | ��طŵ�ʱCl-�ɸ���������Ǩ�� | |
| D�� | �����ᷢ������ӦMg+2H2O�TMg��OH��2+H2�� |


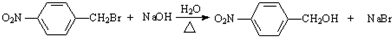 ��
��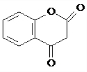 ��
��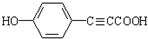 ��
��