��Ŀ����
8����Ҫ��������и�С����1����ͬ��ͬѹ�£���ͬ������NH3��H2S����������Ϊ2��1��
��2���ڱ�״���£�CO������ܶ�Ϊ1.25g/L��
��3���ں���Cu2+��H+��Fe2+��Al3+��Br-��Cl-����Һ�У���ԭ����ǿ�������ǣ�������������������Al3+���������������л�ԭ�Ե�������Fe2+��
��4����������Ϊ98%��Ũ���ᣬ���ܶ�Ϊ1.84g/ml����Ũ��������ʵ���Ũ��Ϊ18.4mol/L���ø���������500ml0.5mol/L��ϡ���ᣬ������Ͳ��ȡ13.6ml��Ũ���ᣬȻ��������ˮϡ�ͣ�
���� ��1��ͬ��ͬѹ�£���������ȵ������ʵ���֮�ȣ�ͬ������NH3��H2S�������Ħ�������ʷ��ȣ�
��2������£�����Ħ�������22.4L/mol�����ݦ�=$\frac{M}{{V}_{m}}$����CO�ܶȣ�
��3�����ʵ�������Խǿ�����ӵĻ�ԭ��Խ����������Խǿ����Ӧ�������ӵ�������Խ������Ԫ�صĻ��ϼ۴����м��̬ʱ���Ⱦ����������־��л�ԭ�ԣ�
��4������c=$\frac{1000�Ѧ�}{M}$�����Ũ��������ʵ���Ũ�ȣ�����ϡ�Ͷ���c1V1=c2V2������ҪŨ����������
��� �⣺��1������n=$\frac{m}{M}$��֪��ͬ������NH3��H2S��������ʵ���֮����Ħ�������ʷ���Ϊ34g/mol��17g/mol=2��1��ͬ��ͬѹ�£��������֮�ȵ��������ʵ���֮�ȣ��ʶ������֮��Ϊ2��1��
�ʴ�Ϊ��2��1��
��2������£�����Ħ�������22.4L/mol���ѣ�CO��=$\frac{M}{{V}_{m}}$=$\frac{28g/mol}{22.4L/mol}$=1.25g/L��
�ʴ�Ϊ��1.25��
��3��±�ص��ʵ�������ǿ��˳��Ϊ��F2��Cl2��Br2��I2���������ӵĻ�ԭ��˳��Ϊ��F-��Cl-��Br-��I-��������Խǿ����Ӧ�������ӵ�������Խ�����������������ļ���������Al3+���������Ӿ�����Ԫ�ص��м�ۣ��������������л�ԭ�ԣ�
�ʴ�Ϊ��Br-��Al3+��Fe2+��
��4���ܶ�Ϊ1.84g/cm3����������Ϊ98%��Ũ��������ʵ���Ũ��Ϊ$\frac{1000��1.84��98%}{98}$mol/L=18.4mol/L������Ҫ����Ũ��������ΪVmL������ϡ�Ͷ��ɣ���18.4 mol/L��VmL=0.5 mol/L��500mL�����V=13.6ml��
�ʴ�Ϊ��18.4mol/L��13.6��
���� ���⿼�鳣�û�ѧ�������йؼ��㡢���ʵ���Ũ���йؼ���ȣ�ע��Թ�ʽ��������������ã���Ŀ�Ѷ��еȣ�

| A�� | Na2O2��Na20��ѧ�����ȶ� | |
| B�� | ����ˮ��Ӧ����NaOH��������Ǽ��������� | |
| C�� | Na20�ܱ�������Na2O2 | |
| D�� | ������H2O��CO2��Ӧ |
| A�� | �ж�������� | B�� | ���Ȼ�۳� | C�� | ��Ӿ | D�� | ��״ |
��1
| ���� | ����ƽ�ⳣ����Ka��Kb�� |
| CH3COOH | 1.8��10-5 |
| HNO2 | 4.6��10-4 |
| HCN | 5��10-10 |
| HClO | 3��10-8 |
| NH3•H2O | 1.8��10-5 |
| �ѣ������� | �ܶȻ�������Ksp�� |
| BaSO4 | 1��10-10 |
| BaCO3 | 2.6��10-9 |
| CaSO4 | 7��10-5 |
| CaCO3 | 5��10-9 |
��1����1�������������У�������������HCN���û�ѧʽ��ʾ����������ʹ������Һ��CH3COOH�ĵ���̶��������볣������IJ�����B������ţ���
A�������¶� B����ˮϡ�� C����������CH3COONa���� D��������������
��2��CH3COONH4��ˮ��Һ�����ԣ�ѡ����ԡ��������ԡ����ԡ�������Һ�и�����Ũ�ȴ�С��ϵ��c��NH4+��=c��CH3COO-����c��OH-��=c��H+����
��3�����ʵ���֮��Ϊ1��1��NaCN��HCN�Ļ����Һ����pH��7������Һ�е���غ��ʽΪc��Na+��+c��H+��=c��CN-��+c��OH-����
��4����ҵ�г���BaSO4ת��ΪBaCO3���ٽ����Ƴɸ��ֿ����Եı��Σ���BaCl2���������������ñ��͵Ĵ�����Һ����BaSO4��ĩ�������ϲ��䴿����BaSO4ת��ΪBaCO3������������BaSO4����Һ���ڸ�����Һ�мӴ����ĩ�����Ͻ��裬ΪʹSO42-���ʵ���Ũ�Ȳ�С��0.01mol•L-1������Һ��CO32-���ʵ���Ũ��Ӧ��0.26mol•L-1��
����֪101kPa��25��ʱ��1mol����������ȫȼ�������ȶ�״̬������ʱ�ų���������
�����£�
| ���� | ���� | ԭú ����Ҫ�ɷ���C�� | ���� ����Ҫ�ɷ�C8H18�� |
| ������kJ�� | 285.8 | 250.9 | 4910 |
��2��H2���Դ���ԭú��������Ϊ����Դ����������ͬ�����£���������H2ȼ�շų�����������ԭú�����ͣ���������Ⱦ����ѭ�����ã�
��3����ҵ�ϵ�ⱥ��ʳ��ˮ�ĸ�����֮һ��H2����Ӧ�����ӷ���ʽ��2Cl-+2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2OH-+H2��+Cl2����
����ͼ��ʾ�����Ṥҵ�в�����SO2ͨ�����й��̼����Ƶ�H2SO4�����Ƶ�H2��
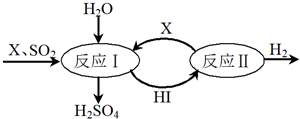
��ش�
��1���ù��̿�ѭ�����õ�������I2��HI��д��ѧʽ����
��2���ù����ܷ�Ӧ�Ļ�ѧ����ʽ��SO2+2H2O=H2SO4+H2��
��3��β���е�SO2����NaOH��Һ���գ�ͬʱ�ɵú�Na2SO3����Ʒ��Ϊ�ⶨ��Ʒ��
Na2SO3��������������ͬѧ���ʵ�����£��гּ�����װ���ԣ���
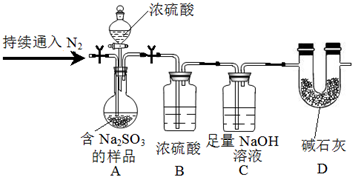
��װ��B�������Ǹ���SO2��
�ڲⶨ��Ʒ��Na2SO3��������������������Ǻ�Na2SO3����Ʒ������װ��Cͨ��SO2ǰ���������
 ��־�������ǣ�������
��־�������ǣ�������| A�� | Ũ���� | B�� | NaCl��Һ | C�� | NH4Cl��Һ | D�� | Na2SO4��Һ |
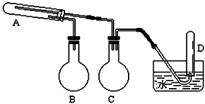 ��һ������������ͨ���ۺ�ˮ������Ӧ�����Եõ����������ʵ���������Լ���ѡ��װ���б�Ҫ������̨�����С���Ȧ��ʯ�����������豸����ͼ�о�����ȥ��������ͼʾ�ж�����˵������ȷ���ǣ�������
��һ������������ͨ���ۺ�ˮ������Ӧ�����Եõ����������ʵ���������Լ���ѡ��װ���б�Ҫ������̨�����С���Ȧ��ʯ�����������豸����ͼ�о�����ȥ��������ͼʾ�ж�����˵������ȷ���ǣ�������| A�� | ʵ�����ʱ�Թ�A��Ӧ������Լ�����ͨ���� | |
| B�� | ��ƿB�������Ƿ�ֹ��������ƿC�������Dz���ˮ���� | |
| C�� | �Թ�D���ռ��õ�����H2 | |
| D�� | 3 mol����Feȫ��ת��ΪFe3O4��ʧȥ8 mol���� |
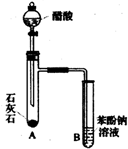 �л�����������еĻ���֮��������Ӱ�죮
�л�����������еĻ���֮��������Ӱ�죮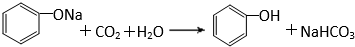 ��
�� 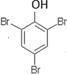 ��
��