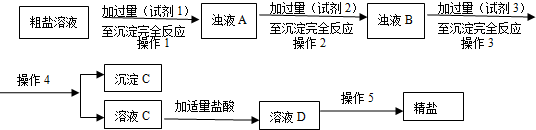
��Ŀ����
8������β���к���NO2��NO��CO���к����壬��ȡ��״����22.4L����β�����о����ָ�����ͨ����ת���������������к�����ɱ���ȫת��Ϊ����N2��CO2����ȡ�����β��ͨ��0.1mol•L-1 40mL NaOH��Һ�У����е�NO2��NOǡ�ñ���ȫ���գ���β����CO�������������Ϊ��˵��������β���������ɷ�������CO��NaOH��Һ������Ӧ������������ת��ΪNaNO2��NaNO3����������| A�� | 0.4% | B�� | 0.7% | C�� | 1% | D�� | 1.3% |
���� β��ͨ���������Ʒ�Ӧʱ��NO2��NOǡ�ñ���ȫ���գ�������Ӧ��NO2+NO+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO2+NaNO3+H2O����Ԫ���غ��֪����ԭ���뵪ԭ�����ʵ�����ȣ���n��NO2+NO��=n��NaOH�����ݴ˼�����������V��NO2+NO����
β��ͨ����ת����ʱ��������Ӧ��4CO+2NO2�TN2+4CO2��2CO+2NO�TN2+2CO2���ɷ���ʽ��֪��ȫ��ΪNO2ʱ��CO��������ȫ��ΪNOʱ��CO�������С����������CO������ټ���CO���������Χ��
��� �⣺������Ӧ��4CO+2NO2�TN2+4CO2��2CO+2NO�TN2+2CO2��
β��ͨ���������Ʒ�Ӧʱ��NO2��NOǡ�ñ���ȫ���գ�������ӦNO2+NO+2NaOH=2NaNO2+H2O��2NO2+2NaOH=NaNO2+NaNO3+H2O����Ԫ���غ��֪����ԭ���뵪ԭ�����ʵ�����ȣ���n��NO2+NO��=n��NaOH��=0.05L��0.1mol/L=0.005mol����V��NO2+NO��=0.005L��22.4L/mol=0.112L��
β��ͨ����ת����ʱ��������Ӧ��4CO+2NO2�TN2+4CO2��2CO+2NO�TN2+2CO2���ɷ���ʽ��֪��
ȫ��ΪNO2ʱ��CO��������CO������Ϊ0.112L��2=0.224L����CO����������ֵΪ��$\frac{0.224L}{22.4L}$��100%=1%��
ȫ��ΪNOʱ��CO�������С��CO�����СΪ0.112L����CO���������СֵΪ��$\frac{0.112L}{22.4L}$��100%=0.5%��
��CO���������Χ����0.5%��1%֮�䣬
��ѡB��
���� ���⿼��������㣬��Ŀ�Ѷ��еȣ��ؼ�������ԭ���غ�������������NO������������ü������з�����𣬲��ؿ���ѧ��������������ѧ����������

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�| A�� | ����������ʳ�� | B�� | ���⡢ţ���Ǽ���ʳ�� | ||
| C�� | ���㡢����������ʳ�� | D�� | ���ס�����Ǽ���ʳ�� |
| A�� | 0.75 mol/L | B�� | 0.25 mol/L | C�� | 0.5 mol/L | D�� | 1 mol/L |
| A�� | KClO3��SO3����ˮ���ܵ��磬��KClO3��SO3Ϊ����� | |
| B�� | Na2SO3��Һ�У�c��H+��+c��HSO3-��+c��H2SO3���Tc��OH-�� | |
| C�� | ��NaAlO2��Һ�еμ�NaHCO3��Һ���г������������� | |
| D�� | ����AgCl�������Һ�м�������ˮʹAgCl�ܽ��ִﵽƽ��ʱ���ڸ��¶���AgCl���ܶȻ����䣬���ܽ��Ҳ���� |
| A�� | N2 | B�� | NH3 | C�� | HCl | D�� | SO2 |
| A�� | $\frac{22.4N}{V}$ mol-1 | B�� | $\frac{VN}{22.4}$mol-1 | C�� | $\frac{VN}{11.2}$ mol-1 | D�� | $\frac{11.2N}{V}$ mol-1 |
 ��2L�ܱ������н��з�Ӧ3Fe��s��+4H2O��g��?Fe3O4��s��+4H2��g����H��0���n��H2O���淴Ӧʱ�䣨t���ı仯��ͼ��ʾ�������ж���ȷ���ǣ�������
��2L�ܱ������н��з�Ӧ3Fe��s��+4H2O��g��?Fe3O4��s��+4H2��g����H��0���n��H2O���淴Ӧʱ�䣨t���ı仯��ͼ��ʾ�������ж���ȷ���ǣ�������| A�� | �÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK=c4��H2O��/c4��H2�� | |
| B�� | 5minʱ�÷�Ӧ�Ħԣ���������9minʱ�Ħԣ��棩 | |
| C�� | 0��5min�ڣ��ԣ�H2��=0.10mol/��L•min�� | |
| D�� | 10minʱƽ�ⷢ���ƶ�������Ͷ�뻹ԭ���������� |
| A�� | Fe������Ϊ2.7g��Al������Ϊ2.8g | B�� | Fe������Ϊ2.8g��Al������Ϊ2.7g | ||
| C�� | Fe������Ϊ5.4g��Al������Ϊ5.6g | D�� | Fe������Ϊ5.6g��Al������Ϊ5.4g |