��Ŀ����
13�� ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ��
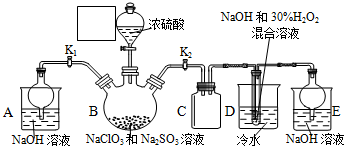
ij��ѧ��ȤС��������ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ����1��ϡ����Ӧ���ڷ�Һ©���У���д�������ƣ���
��2����ʵ��ͨ������A��B��C�������أ��������еĿ����ž����ٹرտ���B������AC�Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ�ž�װ���п��������ɷ�ֹ���ɵ�����������������
��3��ʵ��ʱΪ��ֹ����2������ͨ�����ܽ�������3�У��ɲ�ȡ�Ĵ�ʩ�ǽ����ۻ��������������
��4����FeSO4��Һ�м��루NH4��2SO4������Ʊ�Ħ���ξ��� ����Է�������392�����þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
��Ϊϴ�ӣ�NH4��2SO4•FeSO4•6H2O�ֲ�Ʒ�����з���������ʵ���D
A������ˮϴ B��������ˮϴ��������ˮ�Ҵ�ϴ
C����30%���Ҵ���Һϴ D����90%���Ҵ���Һϴ
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol•L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00mL��ʵ������¼���£�
| ʵ����� | ��һ�� | �ڶ����� | ������ |
| ���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
�ζ��յ�����������һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ$\frac{980c}{a}$��100%������ĸac��ʾ�����ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������BC
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�����
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ
D�������Ը�����ر�Һ����ʱ��������в��ֱ��ʣ�Ũ�Ƚ��ͣ�
���� ����ͼװ���Ʊ��������������۲�����ɫ���ṩ��ѧҩƷ�����ۡ�ϡ���ᡢ����������Һ�����װ�÷�����֪������BC���ر�A����Ҫ������ƿ2�м��������ʣ���Һ©������ϡ���ᣬ���÷�Ӧ���������ž�װ���еĿ����������ռ�C������������Ƿ��Ǵ������������������еĿ����ž���Ȼ��ر�B������AC���������ɵ�������װ��2��ѹǿ��������������Һѹ��װ��3������������Һ�У���Ӧ���ɰ�ɫ������������ɫ�������ɹ۲쵽��������������ɫΪ��ɫ������
��1��������֪��Һ©����Ϊϡ���ᣬ��װ��2�е�����Ӧ��������������������
��2�����װ�÷�����֪������BC���ر�A����Ҫ������ƿ2�м��������ʣ���Һ©������ϡ���ᣬ���÷�Ӧ���������ž�װ���еĿ����������ռ�C������������Ƿ��Ǵ������������������еĿ����ž���Ȼ��ر�B������AC���������ɵ�������װ��2��ѹǿ��������������Һѹ��װ��3������������Һ�У���Ӧ���ɰ�ɫ������������ɫ��������ֹ���ɵ������������������е�����������
��3�������ۻ�����������������Է�ֹ����2������ͨ�����ܽ�������3�У�
��4�������ݸþ����һ���������ȶ������ױ�������������ˮ���������Ҵ�������ϴ�Ӳ����ܽ��Ʒ�����������µ����ʣ�
�ڳ�ȡa g��Ʒ����ˮ�����Ƴ�500mL��Һ����Ũ��Ϊc mol•L-1������KMnO4��Һ�ζ��������ķ�ӦΪ���������Һ������������Ϊ�����ӣ�
��Ӧ�յ���жϿ������ø��������Һ���Ϻ�ɫ�жϣ��������һ����Һ�仯Ϊ�Ϻ�ɫ��������ڲ��䣬֤����Ӧ�ﵽ�յ㣻
���ݷ�Ӧ�Ķ�����ϵ���������������ʵ������õ���NH4��2SO4•FeSO4•6H2O�����������㴿�ȣ����������Һ�������һ�����ϴ�Ӧ��ȥ��
A��ʵ�����ʱ���ӿ̶��߶�ȡ�ζ��յ�ʱ���Ը��������Һ�������Һ�汻��ߣ���ȡ�����С��
B���ζ�ǰ�ζ��ܼ��������ݣ��ζ����������ݲⶨ��Һ�������
C����һ�εζ��õ���ƿ�ô�װҺ��ϴ����������δ��ϴ����װҺ��ϴ���������ĸ��������Һ��������ⶨ�������
D������ʹ�õĸ�����ض���һ���ģ����ĵĸ���������Ӧ����ͬ�ģ�
��� �⣺��1����ʵ��Ŀ�����Ʊ���������������������ϡ���ᷴӦ�Ʊ������������˷�Ӧ��װ��2�н��У�ϡ��������Һ��Ӧʢ���ڷ�Һ©���У���Ӧʢ����1��D�ķ�Һ©���У�
�ʴ�Ϊ����Һ©����
��2����B��C���ر�A������װ��2�в�������������װ��3�еĿ����ž���ʹװ�ô��ڻ�ԭ��Χ��Ȼ��ر�B����A����������ѹǿ���Ѳ�����FeSO4��ѹ�뵽װ��3�У�������ɫ����Fe��OH��2��������������Һ�������е�����������������������������������������ɫ�Ĺ۲�������ţ�����Ҫ�ų�װ���еĿ�������ֹ���ɵ�������������������
�ʴ�Ϊ��B��AC����ֹ���ɵ�������������������
��3��ʵ��ʱΪ��ֹ����2������ͨ�����ܽ�������3�У��ɲ�ȡ�Ĵ�ʩ�ǣ������ۻ�����������������Է�ֹ����2������ͨ�����ܽ�������3�У�
�ʴ�Ϊ�������ۻ��������������
��4������Ϊ���������������ˮ���������Ҵ���Ӧ�����Ҵ�ϴ�ӣ�������������淋��ܽ⣬ͬʱ�����Ҵ���ˮ���ܣ��Ӷ��ﵽϴ�ӵ�Ҫ��ѡD��
�ʴ�Ϊ��D��
�����ø�����ص�ǿ�����ԣ�Fe2+��ǿ��ԭ�ԣ����߷���������ԭ��Ӧ��Fe2+��������Fe3+��1��Mn��+7�ۡ�+2��5����С������5������ԭ�Ӹ���������غ㣬��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O������Һ�еμ��и�����أ�����������Ϻ�ɫ����˵ζ����յ㣺���һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ����Ϊ��һ����ڶ��Ρ����������ϴ��Բ��ƣ����ĸ��������Һ�����Ϊ $\frac{25.02+24.98}{2}$ml=25mL���������ӷ�Ӧ����ʽ���ó���n[��NH4��2SO4•FeSO4•6H2O]=5n��KMnO4��=25��10-3��c��5mol����500mL��Һ�к���n[��NH4��2SO4•FeSO4•6H2O]=25��10-3��c��5��500/25mol=2.5cmol��
������������=2.5c��$\frac{392}{a}$��100%=$\frac{980c}{a}$��100%��
A�����Ӷ����������������ƫС����A����
B���ζ��ܼ��������ݣ��ζ����������ݣ������ĵ�Һ��������ӣ���B��ȷ��
C����ƿ�ô���Һ��ϴ������Һ�����ʵ������ӣ������ĸ�����ص�������ӣ���C��ȷ��
D������ʹ�õĸ�����ض���һ���ģ����ĵĸ���������Ӧ����ͬ�ģ���D����
�ʴ�Ϊ��MnO4-+5Fe2++8H+=Mn2++5Fe3++4H2O�����һ�ε��룬��Һ����ɫ��Ϊdz��ɫ����30s����ɫ��$\frac{980c}{a}$��100%��BC��
���� ���⿼���������������Ʊ��ķdz����Ʊ�������ʵ����̷����жϣ��ζ�ʵ���ע������ͼ���Ӧ�ã�ע�������������ʣ�ʵ����̵�����Ӧ�ã����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| �Ҵ� | ������ | ������ | 1-�嶡�� | |
| �ܶ�/��g•cm-3�� | 0.789 3 | 1.460 4 | 0.809 8 | 1.275 8 |
| �е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��2����1-�嶡��ֲ�Ʒ���ڷ�Һ©���У���ˮ���ã��������²㣨��ϲ㡱���²㡱���ֲ㡱����
��3���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����ABC������ĸ����
A�����ٸ�����ϩ���ѵ����ɡ��� B������Br2������
C������HBr�Ļӷ� D��ˮ�Ƿ�Ӧ�Ĵ���
��4������ȥ������е���������Br2���������������ʺϵ���C������ĸ����
A��NaI B��NaOH C��NaHSO3 D��KCl
��5�����Ʊ�������ʱ�����ñ߷�Ӧ���������ķ�����Ŀ���ǣ�ƽ��������������ķ����ƶ�����Ӧ�������ƶ���
�����Ʊ�1-�嶡��ʱȴ���ܱ߷�Ӧ��������ԭ��1-�嶡����������ķе�����

| A�� | ԭ����������ʵ���Ũ��Ϊ0.1mol•L-1 | |
| B�� | ����ѡ���ɫʯ����Ϊָʾ�� | |
| C�� | �������Ƶ����ʵ���Ũ��Ϊ0.1mol•L-1 | |
| D�� | pHΪ7ʱ������������Ƶ����ʵ���Ϊ0.1mol |
 Ϊ̽���������Ļ������һЩ��ѧ���ʣ�ijѧ��ʵ��С�����������ʵ�飮
Ϊ̽���������Ļ������һЩ��ѧ���ʣ�ijѧ��ʵ��С�����������ʵ�飮