��Ŀ����
11���л���H��ҽҩ���й㷺Ӧ�ã�����ϩΪԭ�Ϻϳ��л���H��·�����£�
��֪��
��

��

��ش��������⣺
��1��D�к��й����������Ȼ���ȩ����д��F�ķ���ʽC9H8O5��D��E�ķ�Ӧ�����Ǽӳɷ�Ӧ��
��2��B��ֻ����һ�ֹ����ţ�A��B��Ӧ�Ļ�ѧ����ʽΪBrCH2-CH2Br+2NaOH $��_{��}^{H_{2}O}$HOCH2-CH2OH+2NaBr��
��3�������йػ�����F������������ȷ����C
A��1molF�������1molNaHCO3��Ӧ
B������Ũ��ˮ����ȡ����Ӧ
C����һ�������£�1molF�������5mol H2�����ӳɷ�Ӧ
D����һ�������£��ܷ������۷�Ӧ
��4��д��һ����G��Ϊͬ���칹�壬�ҷ��������������л���Ľṹ��ʽ
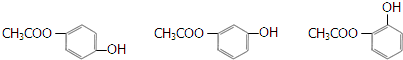 ��
���ٱ�����ֻ������ȡ��������1mol�������������3mol NaOH��Ӧ������FeCl3��Һ����ɫ��
��5��G���Լ�X�ķ�Ӧԭ�����Ʒ�Ӧ�ڣ�д���Լ�X�Ľṹ��ʽCH3COCH3��
���� �������и�����ת����ϵ����ϩ����ӳɵ�AΪBrCH2-CH2Br��B��ֻ����һ�ֹ����ţ�A����������ˮ���BΪHOCH2-CH2OH��B����������Ӧ��CΪOHCCHO��C������D��D�����ӳɷ�Ӧ��E��E����������Ӧ��F������G�ķ���ʽ��֪��F������Ϣ�ٵķ�Ӧ��GΪ ���Ƚ�G��H�Ľṹ��֪��G������Ϣ���еķ�Ӧ��H������XΪCH3COCH3���ݴ˴��⣮
���Ƚ�G��H�Ľṹ��֪��G������Ϣ���еķ�Ӧ��H������XΪCH3COCH3���ݴ˴��⣮
��� �⣺�������и�����ת����ϵ����ϩ����ӳɵ�AΪBrCH2-CH2Br��B��ֻ����һ�ֹ����ţ�A����������ˮ���BΪHOCH2-CH2OH��B����������Ӧ��CΪOHCCHO��C������D��D�����ӳɷ�Ӧ��E��E����������Ӧ��F������G�ķ���ʽ��֪��F������Ϣ�ٵķ�Ӧ��GΪ ���Ƚ�G��H�Ľṹ��֪��G������Ϣ���еķ�Ӧ��H������XΪCH3COCH3��
���Ƚ�G��H�Ľṹ��֪��G������Ϣ���еķ�Ӧ��H������XΪCH3COCH3��
��1������D�Ľṹ��ʽ��֪��D�к��й����������� �Ȼ���ȩ��������F�Ľṹ��ʽ��֪��F�ķ���ʽΪC9H8O5��D��E�ķ�Ӧ�����Ǽӳɷ�Ӧ��
�ʴ�Ϊ���Ȼ���ȩ����C9H8O5���ӳɣ���������
��2��A��B��Ӧ�Ļ�ѧ����ʽΪBrCH2-CH2Br+2NaOH $��_{��}^{H_{2}O}$HOCH2-CH2OH+2NaBr��
�ʴ�Ϊ��BrCH2-CH2Br+2NaOH $��_{��}^{H_{2}O}$HOCH2-CH2OH+2NaBr��
��3�����ݻ�����F�Ľṹ��ʽ��
A��F����һ���Ȼ���1molF�������1molNaHCO3��Ӧ����A��ȷ��
B��F���з��ǻ���λ̼������ԭ�ӣ�����Ũ��ˮ����ȡ����Ӧ����B��ȷ��
C��F����һ��������һ���ʻ�����һ�������£�1molF�������4mol H2�����ӳɷ�Ӧ����C����
D��F�����Ȼ����ǻ�����һ�������£��ܷ������۷�Ӧ����D��ȷ��
�ʴ�Ϊ��C��
��4����G��Ϊͬ���칹�壬�ҷ������������ٱ�����ֻ������ȡ��������1mol�������������3mol�������Ʒ�Ӧ������FeCl3��Һ����ɫ��˵���з��ǻ����������л���Ľṹ��ʽΪ  ��
��
�ʴ�Ϊ�� ��
��
��5����������ķ�����֪��X�Ľṹ��ʽΪCH3COCH3���ʴ�Ϊ��CH3COCH3��
���� ���⿼���л��ƶ������ʣ���Ŀ�Ѷ��еȣ������Ʊ�������ȷ�ϳ�ԭ��Ϊ���ؼ���ע���������ճ����л���ṹ�����ʣ���ȷ������Ӧ��������Ӧԭ������Ӧ���ͣ�����������ѧ�����Ӧ����ѧ֪ʶ��������

 �ס�������ʵ��С������KMnO4������Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ���ѧ����ʽΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+10CO2��+8H2O����
�ס�������ʵ��С������KMnO4������Һ��H2C2O4��Һ��Ӧ�о�Ӱ�췴Ӧ���ʵ����أ���ѧ����ʽΪ��2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+10CO2��+8H2O������1����д���÷�Ӧ��Ӧ�����ӷ���ʽ����ʾ��H2C2O4�����ᣩ��2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O��
���ʵ�鷽�����£�ʵ��������KMnO4��Һ���Ѽ���H2SO4����
��2�����飺ͨ���ⶨ��λʱ��������CO2��������Ĵ�С���Ƚϻ�ѧ��Ӧ���ʵĴ�С��
ʵ��װ����ͼ��ʵ��ʱ��Һ©����A��Һһ���Է��£�A��B�ijɷּ�����
| ��� | T ��K�� | ����������g�� | A��Һ | B��Һ |
| �٢� | 298 | 0 | 2mL 0.2mol/L H2C2O4��Һ | 4mL 0.001mol/L KMnO4��Һ |
| �ڢ� | 298 | 0 | 2mL 0.2mol/L H2C2O4��Һ | 4mL 0.01mol/L KMnO4��Һ |
| �ۢ� | 323 | 0.5 | 2mL 0.2mol/L H2C2O4��Һ | 4mL 0.01mol/L KMnO4��Һ |
| �ܢ� | 298 | 0.5 | 2mL 0.2mol/L H2C2O4��Һ | 4mL 0.01mol/L KMnO4��Һ |
��3�����飺ͨ���ⶨKMnO4��Һ��ɫ����ʱ��Ķ������Ƚϻ�ѧ��Ӧ���ʵĴ�С��ȡ��֧�Թܶ�ʵ��ٺ͢ڷֱ��������ʵ�飬�������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
| ʵ����� | ��Һ��ɫ����ʱ��t��min�� | ||
| ��һ�� | �ڶ��� | ������ | |
| �٢� | 6 | 7 | 7 |
| �ڢ� | 14 | 11 | 14 |
| A�� | ������������H218O��Ϻ���ϡ�����������������ж���������ˮ��ʱ�����й��ۼ��Ķ���������÷���Ӧ��Ϊͬλ��ʾ�ٷ� | |
| B�� | ��Է��������IJⶨ���������ǣ����ӽṹ�ⶨ������Ҫ���ú������ | |
| C�� | C6H5-OH����Ũ��ˮ�������屽�ӣ��ױ�ȴ������Ũ��ˮ��Ӧ��˵�����ӷ��������ڱ���Ӱ�죬ʹ�ǻ���Hԭ�ӱ�û��� | |
| D�� | ȼ�շ����о�ȷ���л���ɷֵ���Ч���� |
| A�� | ��mXa+��nYb-�ã�m+a=n-b | |
| B�� | ��mXa+��nYb-��X��Y��ͬ���� | |
| C�� | X��Yһ������ͬ����Ԫ�� | |
| D�� | ��X�����������ӣ���Y����һ���������� |
| A�� | $\frac{c��O{H}^{-}��}{c��{H}^{+}��}$=1012����Һ��NH4+��Al3+��NO3-��Cl-���Թ��� | |
| B�� | ����þ���������ͻ���� | |
| C�� | ���ۺ���ά�ض����ã�C6H10O5��n��ʾ������Ϊͬ���칹�� | |
| D�� | Ũ��ˮ�еμ�FeCl3������Һ���Ƶ�Fe��OH��3���� |
| A�� | ˮ���������� | B�� | ˮ�����к��зǼ��Լ� | ||
| C�� | �����ȼ���Ļ�ѧ�ɷ���ȫ��ͬ | D�� | ������ˮ�ķ�Ӧ�У�ˮ�������� |
| A�� | ����-20���ɻ��ϴ���ʹ�õ�̼��ά��һ�����͵��л��߷��Ӳ��� | |
| B�� | ���Ͻ�Ĵ���ʹ������Ϊ�������������Ȼ�ԭ�����������л�ȡ�� | |
| C�� | ���ָʾ����ɫ��ú��Һ������ˮ���塢������Ӧ���漰��ѧ�仯 | |
| D�� | ����þ�Ż��ʹ�øɷ��������𣬵���������ĭ�������� |

| A�� | 1mol X����5mol NaOH��Ӧ | B�� | X�ܷ����ӳɡ�ȡ����������Ӧ | ||
| C�� | X�ķ���ʽΪC7H8O6 | D�� | X�����ᡢ�Ҵ����ܷ���ȡ����Ӧ |
| A�� | 28g CO��C2H4�������ķ�������NA�����ԼΪ22.4L | |
| B�� | 9.2g������Ͷ�뵽��������ˮ�����������к���0.4NA���� | |
| C�� | 25��ʱ��pH=13��1.0L Ba��OH��2��Һ�к��е�OH-��ĿΪ0.2NA | |
| D�� | 0.1mol/LFeCl3��Һ������Fe3+����ĿС��0.1NA |