��Ŀ����
ʵ����Ҫ����500mL 0.1mol?L-1Na2CO3��Һ���ش��������⣮
��1��Ӧ��������ƽ��ȡʮˮ��̼���ƾ��� g��
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ƽƽ��ʱʵ�ʳ�����̼���ƾ����� g����1g���������룩
��3����������ƽ��С�ձ��Ƴ�̼���ƾ��������������ȷ����˳��ı��Ϊ ������������ö�Σ���
A��������� B�������������̶ȳߵ���̶ȴ�
C����̼���ƾ������С�ձ��г��� D�������յ�С�ձ�
E��������Ż�������� F����¼�������
��4������Na2CO3��Һʱ���õ���Ҫ������ ��
��5����ʵ���������������Һ��Ũ����ƫ�ߡ�ƫ�ͻ��Dz��䣿
A����ˮʱ�����̶��� ��
B�����ǽ�ϴ��Һ��������ƿ ��
C������ƿ�ڱڸ���ˮ���δ���и��ﴦ�� ��
D�����ݺ�ҡ�ȣ�Һ����ڿ̶��� ��
��1��Ӧ��������ƽ��ȡʮˮ��̼���ƾ���
��2�����ڳ�����Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ƽƽ��ʱʵ�ʳ�����̼���ƾ�����
��3����������ƽ��С�ձ��Ƴ�̼���ƾ��������������ȷ����˳��ı��Ϊ
A��������� B�������������̶ȳߵ���̶ȴ�
C����̼���ƾ������С�ձ��г��� D�������յ�С�ձ�
E��������Ż�������� F����¼�������
��4������Na2CO3��Һʱ���õ���Ҫ������
��5����ʵ���������������Һ��Ũ����ƫ�ߡ�ƫ�ͻ��Dz��䣿
A����ˮʱ�����̶���
B�����ǽ�ϴ��Һ��������ƿ
C������ƿ�ڱڸ���ˮ���δ���и��ﴦ��
D�����ݺ�ҡ�ȣ�Һ����ڿ̶���
���㣺��Һ������
ר�⣺ʵ����
��������1������m=nM=cvM���㣻
��2��������ƽƽ��ԭ������������=��������+�������ݣ��ݴ˼��㣻
��3����������ƽ��������ʱ����ȷ����˳����з������
��4������ʵ������IJ�����ȷ����Һ��������������
��5��������������Һ���ʵ�������Һ�����Ӱ�죬����c=
����������������ҺŨ�ȵ�Ӱ�죮
��2��������ƽƽ��ԭ������������=��������+�������ݣ��ݴ˼��㣻
��3����������ƽ��������ʱ����ȷ����˳����з������
��4������ʵ������IJ�����ȷ����Һ��������������
��5��������������Һ���ʵ�������Һ�����Ӱ�죬����c=
| n |
| V |
���
�⣺��1��ʵ��������500mL0.1mol/LNa2CO3��Һ��ҪNa2CO3?10H2O������Ϊ��0.5L��0.1mol/L��286g/mol=14.3g��
�ʴ�Ϊ��14.3��
��2��������Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ʵ�ʳ�����̼���ƾ�����14g-0.3g=13.7g��
�ʴ�Ϊ��13.7��
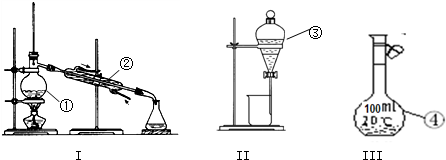
��3������ƽ��������ʱ����ȷ���������ǣ��Ƚ����������̶ȳߵ���̶ȴ�������㣬Ȼ���ȳ����յ�С�ձ�����������¼�����Ľ������̼���ƾ������С�ձ��г�������¼�����Ľ����������Ż�������ڣ�������������̶ȳߵ���̶ȴ����ʴ�Ϊ��BADFCFEB��
��4����Һ���Ʋ��������У��������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡҩƷ�����ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500ml����ƿ�У����ò�����������ϴ�Ӳ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����Ҫ������Ϊ���ձ�����ƽ����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
�ʴ�Ϊ���ձ�����ƽ����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
��5��A������ʱ��ˮʱ�����̶��ߣ���Һ���ƫ������Ũ��ƫ�ͣ�
B��ϴ��Һδ��������ƿ�������������ʧ��ʹ������ҺŨ��ƫ�ͣ�
C������ƿ�ڱڸ���ˮ���δ��������ʺ��ܼ�����Ӱ�죬��������Ũ�Ȳ��䣻
D�����ݺ�ҡ�ȣ�Һ����ڿ̶��ߣ�����ƿ�ڱڸ���ˮ�飬��������ҺŨ�Ȳ��䣻
�ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ����䣻���䣮
�ʴ�Ϊ��14.3��
��2��������Ʒʱ��ҩƷ������ƽ�����ϣ����������ƽ�����ϣ���ʵ�ʳ�����̼���ƾ�����14g-0.3g=13.7g��
�ʴ�Ϊ��13.7��
��3������ƽ��������ʱ����ȷ���������ǣ��Ƚ����������̶ȳߵ���̶ȴ�������㣬Ȼ���ȳ����յ�С�ձ�����������¼�����Ľ������̼���ƾ������С�ձ��г�������¼�����Ľ����������Ż�������ڣ�������������̶ȳߵ���̶ȴ����ʴ�Ϊ��BADFCFEB��
��4����Һ���Ʋ��������У��������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡҩƷ�����ձ����ܽ⣬���ò��������裬��ȴ��ת�Ƶ�500ml����ƿ�У����ò�����������ϴ�Ӳ�����ϴ��Һ��������ƿ�У�����ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����Ҫ������Ϊ���ձ�����ƽ����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
�ʴ�Ϊ���ձ�����ƽ����������500mL����ƿ����ͷ�ιܡ�ҩ�ף�
��5��A������ʱ��ˮʱ�����̶��ߣ���Һ���ƫ������Ũ��ƫ�ͣ�
B��ϴ��Һδ��������ƿ�������������ʧ��ʹ������ҺŨ��ƫ�ͣ�
C������ƿ�ڱڸ���ˮ���δ��������ʺ��ܼ�����Ӱ�죬��������Ũ�Ȳ��䣻
D�����ݺ�ҡ�ȣ�Һ����ڿ̶��ߣ�����ƿ�ڱڸ���ˮ�飬��������ҺŨ�Ȳ��䣻
�ʴ�Ϊ��ƫ�ͣ�ƫ�ͣ����䣻���䣮
���������⿼��һ�����ʵ���Ũ����Һ�����Ʋ������������ȣ��Ѷ��еȣ�ע�����c=
������Һ��ԭ������������
| n |
| V |

��ϰ��ϵ�д�
�����Ŀ
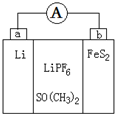 ��ͼ��һ��Ӧ�ù㷺��﮵�أ�LiPF6�ǵ���ʣ�SO��CH3��2���ܼ�����Ӧԭ����4Li+FeS2�TFe+2Li2S������˵����ȷ���ǣ�������
��ͼ��һ��Ӧ�ù㷺��﮵�أ�LiPF6�ǵ���ʣ�SO��CH3��2���ܼ�����Ӧԭ����4Li+FeS2�TFe+2Li2S������˵����ȷ���ǣ�������| A����װ�ý�����ת��Ϊ��ѧ�� |
| B��Li+��a���ƶ� |
| C��������ˮ����SO��CH3��2���ܼ� |
| D��b����Ӧʽ��FeS2+4Li++4e-�TFe+2Li2S |