��Ŀ����
6������Ԫ�ػ�������ҽҩ�����ײ����Ʊ���Ӧ�ù㷺����1��PԪ�صĻ�̬���ӵ����Ų�ʽΪ1s22s22p63s23p3
��2�����ķ���ʽΪP4����ṹ��ͼ1��ʾ����ѧ��Ŀǰ�ϳ��� N4���ӣ�N ԭ�ӵ��ӻ����������sp3��N-N-N ���ļ���Ϊ60�㣻

��3��N��P��Asԭ�ӵĵ�һ�������ɴ�С��˳��ΪN��P��As��
��4��HNO3��HNO2�ǵ�Ԫ���γɵĺ����ᣬ���Խ�ǿ����HNO3�������û�й¶Ե��ӵ���NO3-��NO2-�۵��ӹ�����ƽ�������Σ�
��5��������������Ľṹ��ͼ2��ʾ��N��As��ͬ��Ԫ�أ�B��Ga��ͬ��Ԫ�أ������黯�ؾ�����������������ṹ���ƣ����־������۵�ϸߵ��ǵ����������黯�ؾ���ľ����߳�Ϊa pm�������ܶ�Ϊ$\frac{5.8��1{0}^{32}}{{N}_{A}��{a}^{3}}$g•cm-3���ú�a��ʽ�ӱ�ʾ����NAΪ�����ӵ�������ֵ����
���� ��1��Pԭ�Ӻ�����15�����ӣ����ݹ���ԭ����д��̬Pԭ�Ӻ�������Ų�ʽ��
��2��N4������ÿ��Nԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ���ӻ���ʽ���÷��ӽṹ�Ͱ����ӽṹ��ͬ��Ϊ��������ṹ��
��3��ͬһ����Ԫ�أ�Ԫ�ص�һ����������ԭ�������������С��
��4��ͬһ�ǽ���Ԫ���γɵĺ������У����ǻ�Oԭ�Ӹ���Խ���������Խǿ����������з��ǻ���ԭ�Ӹ������������ᣬ�����������Խ�ǿ������������йµ��ӶԸ���=$\frac{5+1-3��2}{2}$=0��������������йµ��ӶԸ���=$\frac{5+1-2��2}{2}$=1������������Ӽ۲���ӶԸ���=2+$\frac{5+1-2��2}{2}$=3��
��5�������黯�ؾ�����������������ṹ���ƣ�������ԭ�Ӿ��壬ԭ�Ӱ뾶ԽС�����ۼ�Խǿ�������۵�Խ�ߣ����ݾ�̯�����㾧����As��Gaԭ����Ŀ����ʾ���������������ݦ�=$\frac{m}{V}$���㾧���ܶȣ�
��� �⣺��1��Pԭ�Ӻ�����15�����ӣ����ݹ���ԭ����д��̬Pԭ�Ӻ�������Ų�ʽΪ1s22s22p63s23p3��
�ʴ�Ϊ��1s22s22p63s23p3��
��2��N4������ÿ��Nԭ�Ӽ۲���ӶԸ�����4�Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������ж�Nԭ���ӻ���ʽΪsp3���÷��ӽṹ�Ͱ����ӽṹ��ͬ��Ϊ��������ṹ������Ϊ60�㣬
�ʴ�Ϊ��sp3��60�㣻
��3��ͬ�������϶��µ�һ�����ܼ�С���ʵ�һ�����ܣ�N��P��As��
�ʴ�Ϊ��N��P��As��
��4��ͬһ�ǽ���Ԫ���γɵĺ������У����ǻ�Oԭ�Ӹ���Խ���������Խǿ����������з��ǻ���ԭ�Ӹ������������ᣬ�����������Խ�ǿ��NO3-�йµ��ӶԸ���=$\frac{5+1-3��2}{2}$=0��������������йµ��ӶԸ���=$\frac{5+1-2��2}{2}$=1������������Ӽ۲���ӶԸ���=2+$\frac{5+1-2��2}{2}$=3��NO2-�۵��ӹ�����ƽ�������Σ�
�ʴ�Ϊ��HNO3��NO3-��ƽ�������Σ�
��5�������黯�ؾ�����������������ṹ���ƣ�������ԭ�Ӿ��壬ԭ�Ӱ뾶N��As��B��Ga���ʵ������й��ۼ���ǿ��������ľ����۵���ߣ�������As��Gaԭ����Ŀ��Ϊ4����������Ϊ4��$\frac{145}{{N}_{A}}$g�������ܶ�Ϊ4��$\frac{145}{{N}_{A}}$g�£�a��10-10 cm��3=$\frac{5.8��1{0}^{32}}{{N}_{A}��{a}^{3}}$g��cm-3��
�ʴ�Ϊ��������$\frac{5.8��1{0}^{32}}{{N}_{A}��{a}^{3}}$��
���� �����Ƕ����ʽṹ�����ʵĿ��飬�漰��������Ų����ӻ���ʽ�жϡ������ܡ������ṹ����㡢�۷е�Ƚϵȣ���5��Ϊ�״��㡢�ѵ㣬��Ҫѧ���߱�һ���Ŀռ�������Ŀ�Ѷ��еȣ�

��CO��H2�Ƽ״��ķ�Ӧ��CO��g��+2H2��g��?CH3OH ��g����H=-99kJ•mol-1
| ��ѧ�� | H-H | C-O | C��O | H-O | C-H |
| ����/kJ��mol-1 | a | b | x | c | d |
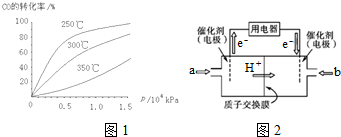
��2�����ݻ�Ϊ1L�ĺ��������У��ֱ��о���T1��T2��T3�����¶��ºϳɼ״��Ĺ��ɣ����������¶��²�ͬ��H2��CO����ʼ��ɱȣ���ʼʱCO�����ʵ�����Ϊ1mol����COƽ��ת���ʦ���CO���Ĺ�ϵ��ͼ1��ʾ��
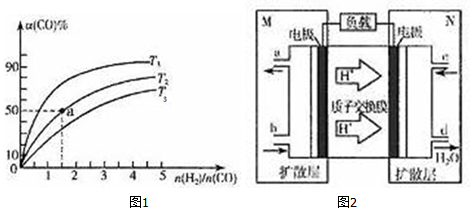
��T1��T2��T3�У��¶���ߵ���T3��
������ͼ��a���Ӧ�����ݣ�����÷�Ӧ��T2�¶��µ�ƽ�ⳣ��K=4L2•mol-2��
���ı�����c������ţ�����ʹK=6L2•mol-2��
a������ѹǿ b����Ӧ���Ũ��
c�������¶� d����С$\frac{n��{H}_{2}��}{n��CO��}$
��3���ü״���ȼ�ϵ�أ��乤��ԭ����ͼ2��ʾ��
��M��������Ӧ�ĵ缫��ӦʽCH3OH+H2O-6e-=CO2+6H+��
��ά�ֵ���ǿ��Ϊ0.5A����ع���10���ӣ����������ļ״�$\frac{0.5��600}{96500��6}��32$g������֪F=96500C•mol-1��д���������ʽ���ɣ�
 ��ij�ݻ�һ�����ܱ������У����淴ӦA��g��+B��g��?xC��g������H��0��������ͼ��I����ʾ��ϵ���ɴ��ƶ϶�ͼ��II������ȷ˵���ǣ�������
��ij�ݻ�һ�����ܱ������У����淴ӦA��g��+B��g��?xC��g������H��0��������ͼ��I����ʾ��ϵ���ɴ��ƶ϶�ͼ��II������ȷ˵���ǣ�������| A�� | p3��p4��Y���ʾA��ת���� | |
| B�� | p3��p4��Y���ʾ���������ܶ� | |
| C�� | p3��p4��Y���ʾB��Ũ�� | |
| D�� | p3��p4��Y���ʾ��������ƽ��Ħ������ |
| A�� | 32gO2��32gO3����ԭ����Ŀ��Ϊ2NA | |
| B�� | ��״���£�11.2 Lˮ�к��е�ԭ������1.5NA | |
| C�� | 0.1 mol Fe���뻯ѧ��Ӧת�Ƶĵ�����һ��Ϊ0.3NA | |
| D�� | ��ͬ��ͬѹ�£���ͬ������κ����嵥��������ԭ������� |
| A�� | 84% | B�� | 24% | C�� | 19.6% | D�� | 42% |
| A�� | �����������ȷֽ�ʱ����ȼ������������ | |
| B�� | ���������ֽ����ɵ�ˮ����ϡ��ȼ������������ | |
| C�� | �����������ȷֽ���������ʹ��ȼ����Ż�㽵�� | |
| D�� | ���������ֽ����ɵ�������������ȼ������棬ʹȼ������O2���� |