��Ŀ����
8��ԭ������С��36��X��Y��Z��W��J����Ԫ�أ�ԭ������������������XԪ��ԭ�Ӱ뾶������Ԫ������С�ģ�YԪ�غ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ��WԪ��ԭ���������������ڲ��������3����JԪ��ԭ������Ϊ24����Ԫ�ط��Ż�ѧʽ��ʾ������1��Y��Z��W�ĵ�һ��������С�����˳��ΪC��O��N
��2��JԪ��ԭ�ӵ���Χ�����Ų�Ϊ3d54s1
��3��Y2X2������Yԭ�ӹ�����ӻ�����Ϊsp
��4��Y��W�γɵ�һ��YW���ӣ���÷��ӻ�Ϊ�ȵ�����ķ��Ӻ�������ΪN2��CN-����дһ�֣�
��5��JCl3����Z��W���⻯���γ���λ��Ϊ6����������Ӧ��������ʵ���֮��Ϊ2��1��������ȫ��λ����磮��������Ļ�ѧʽΪ��[Cr��NH3��4��H2O��2]Cl3��
���� ԭ������С��36��X��Y��Z��W��J����Ԫ�أ�ԭ������������������XԪ��ԭ�Ӱ뾶������Ԫ��ԭ������С�ģ���XΪHԪ�أ�YԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ����Yԭ�Ӻ�������Ų�Ϊ1s22s22p2��YΪCԪ�أ�WԪ��ԭ���������������ڲ��������3����W��2�����Ӳ㣬����������Ϊ6����WΪOԪ�أ�Z��ԭ����������̼Ԫ������Ԫ��֮�䣬��ZΪNԪ�أ�JԪ��ԭ������Ϊ24��JΪCrԪ�أ��ݴ˽��
��� �⣺ԭ������С��36��X��Y��Z��W��J����Ԫ�أ�ԭ������������������XԪ��ԭ�Ӱ뾶������Ԫ��ԭ������С�ģ���XΪHԪ�أ�YԪ��ԭ�Ӻ��������ֲ�ͬ���ܼ��Ҹ����ܼ������ĵ�������ͬ����Yԭ�Ӻ�������Ų�Ϊ1s22s22p2��YΪCԪ�أ�WԪ��ԭ���������������ڲ��������3����W��2�����Ӳ㣬����������Ϊ6����WΪOԪ�أ�Z��ԭ����������̼Ԫ������Ԫ��֮�䣬��ZΪNԪ�أ�JԪ��ԭ������Ϊ24��JΪCrԪ�أ�
��1��ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ��һ��������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������C��O��N��
�ʴ�Ϊ��C��O��N��
��2��JΪCrԪ�أ����������Ϊ24��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1����Χ�����Ų�Ϊ��3d54s1��
�ʴ�Ϊ��3d54s1��
��3��Y2X2����ΪC2H2���ṹʽΪH-C��C-H��������Cԭ�ӳ�1��C-H����1��C��C�����ӻ������ĿΪ2��Cԭ�ӹ�����ӻ�����Ϊsp��
�ʴ�Ϊ��sp��
��4��Y��W�γɵ�һ��NO���ӣ���÷��ӻ�Ϊ�ȵ�����ķ��Ӻ�������ΪN2��CN-��
�ʴ�Ϊ��N2��CN-��
��5��CrCl3����NH3��H2O�γ���λ��Ϊ6����������Ӧ������������ʵ���֮��Ϊ2��1��������ȫ��λ����磬��������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ��[Cr��NH3��4��H2O��2]Cl3��
���� ���⿼���������Ų����ɡ��ӻ�������ȵ����塢�����ȣ��Ѷ��еȣ��ƶ�Ԫ���ǽ���Ĺؼ���

| A�� |  ����-OH ����-OH | B�� |  ����-CHO ����-CHO | ||
| C�� | CH3-O-CH3 ���� | D�� |  ȩ��-CHO ȩ��-CHO |
| A�� | ���������Һ��ͨ�����������̼��Ca2++2ClO-+H2O+CO2�TCaCO3��+2HClO | |
| B�� | NaHCO3��Һ�м���������Ba��OH��2��Һ��HCO3-+Ba2++OH-�TBaCO3��+H2O | |
| C�� | �ƺ�ˮ��Ӧ��Na+H2O�TNa++OH-+H2�� | |
| D�� | ��������ˮ��Cl2+H2O�T2H++Cl-+ClO- |
| A�� | CH4��C2H4 | B�� | BF3��NH3 | C�� | BeCl2��SCl2 | D�� | H2O��H2S |
| A�� | �ǽ���������һ�����ڹ��ۼ� | |
| B�� | �����ۼ��Ļ�����һ���ǹ��ۻ����� | |
| C�� | �����Ӽ��Ļ�����һ�������ӻ����� | |
| D�� | ����ˮ�ܵ���Ļ�����������ӻ����� |
| ѡ�� | ʵ����ʵ | ���۽��� |
| A | Nԭ�ӵĵ�һ������С��Oԭ�� | ͬ����Ԫ��ԭ�ӵĵ�������ԭ��������������� |
| B | CO2Ϊֱ���η��� | CO2������CΪsp�ӻ�������Ϊ180�� |
| C | ���ʯ���۵����ʯī | ���ʯ��ԭ�Ӿ��壬ʯī�Ƿ��Ӿ��� |
| D | HF�ķе����HCl | HF����Է�������С��HCl |
| A�� | A | B�� | B | C�� | C | D�� | D |

 ��
��


 ��֪ A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�FԪ�صĻ�̬ԭ�ӵ����ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�
��֪ A��B��C��D��ԭ������������������ֶ���������Ԫ�أ�A������������������������Bԭ�ӵļ۵����Ų�Ϊnsnnpn��D�ǵؿ��к�������Ԫ�أ�E�ǵ������ڵ�p��Ԫ���������ֻ��2�ԳɶԵ��ӣ�FԪ�صĻ�̬ԭ�ӵ����ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�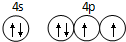 ��
��