��Ŀ����
3����֪Ca3��PO4��2��SiO2��C���¹��ȿ��Է�Ӧ�õ�CaSiO3��P4������CO����Ӧ���£�Ca3��PO4��2+SiO2+C$\stackrel{����}{��}$CaSiO3+P4��+CO��
��1����ƽ�÷�Ӧ����ʽ�����������ת�Ʒ������Ŀ��
2Ca3��PO4��2+6SiO2+10C$\stackrel{����}{��}$6CaSiO3+1P4��+10CO��
��2���÷�Ӧ�б���ԭ��Ԫ����p������������CO��
��3��ÿ����24.0g̼ʱ����4NA�����ӷ���ת�ƣ�����P424.8g��
��4����Ӧ���û�����壬����ɱ���£��������ܶ�Ϊ1.64g/L��������λС��������÷�Ӧ���漰������Ԫ����ͬ����ĵ�Ԫ�أ������ˮ�帻Ӫ��������Ҫԭ����ij��ˮ��NH4Cl����Ϊ180mg/L��
��5��д��NH4Cl�ĵ���ʽ
 ��
����6��Ϊ��ȥ��ˮ�е�NH4+����103L����ˮ�м���0.1mol/LNaOH��Һ���������·�Ӧ��NH4++OH-��NH3+H2O������������ҪNaOH��Һ�����Ϊ33.64L��������������λС����
���� ��1���ڴ˷�Ӧ��Ca3��PO4��2��PԪ�ؼ�̬��+5���͵�0�۵�P4�����ϼ۽�����20��C��0�����ߵ�+2�۵�CO�����ϼ�������+2�ۣ���С������Ϊ20����P4ǰϵ��Ϊ1��COǰϵ��Ϊ10��������ԭ���غ������ƽ��
��2����Ca3��PO4��2��PԪ�صĻ��ϼ���+5�۽���Ϊ0�����ϼ۽��ͣ���Ϊ�����������������������Ա���ԭ�õ����ǻ�ԭ����P4������
��3�����Ԫ�ػ��ϼ۵ı仯�������غ������㣻
��4���ݦ�=$\frac{m}{V}$���㣻
��5���Ȼ��Ϊ���Ӿ��壬�ɰ���������������ͨ�����Ӽ������һ��
��6���� NH4Cl����������NaOH��
53.5g 1mol
180mg/L��103 L��103 g/mg 0.1mol/L��V����֮�ã���ˮ�еİ������������ߣ�������NH3•H2O?NH3+H2O��ƽ�������ƶ���
��� �⣺��1���ڴ˷�Ӧ��Ca3��PO4��2��PԪ�ؼ�̬��+5���͵�0�۵�P4�����ϼ۽�����20��C��0�����ߵ�+2�۵�CO�����ϼ�������+2�ۣ���С������Ϊ20����P4ǰϵ��Ϊ1��COǰϵ��Ϊ10��������ԭ���غ���ƽ���ʷ���ʽ2Ca3��PO4��2+6SiO2+10C$\stackrel{����}{��}$6CaSiO3+P4��+10CO���ʷ���ʽǰ���ϵ��Ϊ��2��6��10��6��1��10�������ű�ʾ
�ʴ�Ϊ��2��6��10��6��1��10��
��2����Ca3��PO4��2��PԪ�صĻ��ϼ���+5�۽���Ϊ0�����ϼ۽��ͣ�����������������Ϊ���������������Ա���ԭ����PԪ�ر���ԭ��CԪ����0�����ߵ�+2�۵�
CO�����ϼ����ߣ�����ԭ�����õ���CO���������
�ʴ�Ϊ��P��CO��
��3����3��ÿ����24.0g̼ʱ��ת�Ƶ���Ϊ$\frac{24g}{12g/mol}$����2-0��=4mol��N=nNA=4NA���ɷ�Ӧ��֪������10molC����1molP4��������Ϊ0.2mol��124g/mol=24.8g
�ʴ�Ϊ��4NA��24.8��
��4����4���ɷ�Ӧ��֪��������������Ϊ24.8g+2mol��28g/mol=80.8g����������Ϊ2.2mol��22.4L/mol=49.28L�������ܶ�Ϊ$\frac{80.8g}{49.28g/L}$=1.64g/L
�ʴ�Ϊ��1.64
��5���Ȼ�������ӻ�����ɰ��������������ӹ��ɣ�����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��6���� NH4Cl����������NaOH��
53.5g 1mol
180mg/L��103 L��103 g/mg 0.1mol/L��V��
$\frac{53.5}{180}$=$\frac{1}{0.1V}$��֮�ã�V=33.64L
�ʴ�Ϊ��33.64��
���� ���⿼��������ԭ��Ӧ�����㣬��ȷ��Ӧ��Ԫ�صĻ��ϼ۱仯�ǽ����Ĺؼ�����4��Ϊ������ѵ㣬��Ŀ�Ѷ��еȣ�

| A�� | ����ֽ�Խ��ȫ�������������Խ�� | |
| B�� | ������0.1mol������̼���������Ȼ���Ϊ5.85g | |
| C�� | ������̼�����ƺ���Խ�࣬��Ԫ�صĺ���ҲԽ�� | |
| D�� | ��Һ�е�������Ϊ0.11mol��ԭ�����е�̼������Ϊ8.4g |
| A�� | ������Ӧ | B�� | ȡ����Ӧ | C�� | �ӳɷ�Ӧ | D�� | ��ȥ��Ӧ |
| ���� | ��; | |
| A | Һ���������� | ����������� |
| B | NH4HCO3�����ֽ� | ���������� |
| C | ������������HF��Ӧ | ���������ά |
| D | ����������ʹ��ˮ��ɫ | ������Ư�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ��ͼ�ǽ���þ��±�ط�Ӧ�������仯ͼ����Ӧ����������Ϊ298Kʱ���ȶ�״̬��������ѡ���в���ȷ���ǣ�������
��ͼ�ǽ���þ��±�ط�Ӧ�������仯ͼ����Ӧ����������Ϊ298Kʱ���ȶ�״̬��������ѡ���в���ȷ���ǣ�������| A�� | Mg��F2��Ӧ������� | |
| B�� | MgF2��s��+Br2��l���TMgBr2��s��+F2��g������ | |
| C�� | MgBr2��Cl2��Ӧ���� | |
| D�� | ����������ȶ�˳��MgI2��MgBr2��MgCl2��MgF2 |
| A�� | ���к��������ױ� | B�� | �Ҵ��к����������� | ||
| C�� | �屽�к��������� | D�� | ���������к����������� |
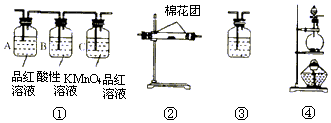 ��1��д��Ũ������ľ̿���ڼ��������·�Ӧ�Ļ�ѧ����ʽ�����������ת�ƹ�ϵ
��1��д��Ũ������ľ̿���ڼ��������·�Ӧ�Ļ�ѧ����ʽ�����������ת�ƹ�ϵ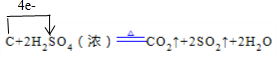 ��
�� ��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������ɼ����к��ȣ��ش��������⣺
��50mL0.50mol/L������50mL0.55mol/LNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų������ɼ����к��ȣ��ش��������⣺