��Ŀ����
�ߴ��������������Ƹ�ѹ�ƵƵ��մɹܣ�ʵ������ȡ�ߴ����������������£�

��1�������ӡ������Ǽ��������������⣬�ð�ˮ������Һ��pHΪ8.0���Գ�ȥ�������Һ�е�����Fe2+������Fe2+�ѳ�����ʵ������������� ��
��2�������£�KSP[Fe��OH��3]=4.0��10-38���������ϼ�����Ӻ���Һ��c��Fe3+������ ��
��3��������������Һʱ�����������ữ��Ŀ���� ��
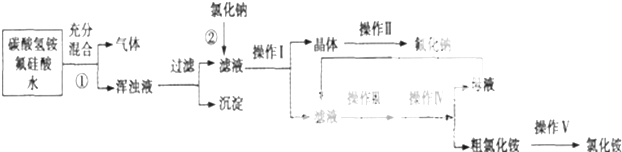
��4����������ͼ����ᾧ���ⲽ�����У�ĸҺ�� �õ�����������ᾧˮ��
��5����������ͼ������롱�������Ӧ�������� ������ĸ���ţ�
A������B����ҺC������D��ϴ�Ӿ��壮
��6���ⶨ�������ɵķ�����
a����ȡ0.4530g�������Ʒ���������գ���Al2O3����0.0510g��
b����ȡ0.4530g�������Ʒ������������ˮ�ܽ⣬�ټ����Թ�����BaCl2��Һ�����»������ˡ��һ�����BaSO4 0.4660g��
c����ȡ0.4530g�������Ʒ������������NaOH��Һ�����ȣ�����������������ͨ����ʯ�Һ�Ũ���ᣬŨ��������0.0170g��
��������Ļ�ѧʽΪ ��

��1�������ӡ������Ǽ��������������⣬�ð�ˮ������Һ��pHΪ8.0���Գ�ȥ�������Һ�е�����Fe2+������Fe2+�ѳ�����ʵ�������������
��2�������£�KSP[Fe��OH��3]=4.0��10-38���������ϼ�����Ӻ���Һ��c��Fe3+������
��3��������������Һʱ�����������ữ��Ŀ����
��4����������ͼ����ᾧ���ⲽ�����У�ĸҺ��
��5����������ͼ������롱�������Ӧ��������
A������B����ҺC������D��ϴ�Ӿ��壮
��6���ⶨ�������ɵķ�����
a����ȡ0.4530g�������Ʒ���������գ���Al2O3����0.0510g��
b����ȡ0.4530g�������Ʒ������������ˮ�ܽ⣬�ټ����Թ�����BaCl2��Һ�����»������ˡ��һ�����BaSO4 0.4660g��
c����ȡ0.4530g�������Ʒ������������NaOH��Һ�����ȣ�����������������ͨ����ʯ�Һ�Ũ���ᣬŨ��������0.0170g��
��������Ļ�ѧʽΪ
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�Ʊ�ʵ�鷽�������
ר�⣺ʵ�������
�����������������Һ���ӣ�����PH��ȥ�����ӣ����˵õ���Һ����Һ�м�����������Һ�ϳ�������ͨ�� ����Ũ������ȴ�ᾧ������ϴ�ӵõ����壬���յõ���Ʒ��
��1��ȡ����������H2O2��Һ���μ�KSCN��Һ��������������˵��Fe2+�ѳ�����
��2�����������������ܶȻ���PH=8�����������Ũ�ȣ�
��3������������Һ���ܹ�����ˮ����������������
��4�����ɻ��������������ʵ�������������������Ũ������ȴ�ᾧ������ϴ�ӵõ����壻
��5������������Һ����IJ����ǹ��ˣ�
��6�������������ݣ��������Ԫ������������������������ٸ��������������ˮ���������������������Ļ�ѧʽ��
��1��ȡ����������H2O2��Һ���μ�KSCN��Һ��������������˵��Fe2+�ѳ�����
��2�����������������ܶȻ���PH=8�����������Ũ�ȣ�
��3������������Һ���ܹ�����ˮ����������������
��4�����ɻ��������������ʵ�������������������Ũ������ȴ�ᾧ������ϴ�ӵõ����壻
��5������������Һ����IJ����ǹ��ˣ�
��6�������������ݣ��������Ԫ������������������������ٸ��������������ˮ���������������������Ļ�ѧʽ��
���
�⣺��1������Fe2+�Ƿ������ʵ������ǣ�ȡ�������Ӻ����Һ��������H2O2��Һ�����μ�KSCN��Һ��������������˵��Fe2+�ѳ�����
�ʴ�Ϊ��ȡ������Һ���Թ��У��ữ�����μӼ�������KMnO4��Һ��������������˵��Fe2+�ѳ�������ȡ�������Ӻ����Һ��������H2O2��Һ�����μ�KSCN��Һ��������������˵��Fe2+�ѳ�����
��2��KSP[Fe��OH��3]=4.0��10-38��PH=8����Һ������������Ũ��Ϊ��1��10-6mol/L��c��Fe3+��=
mol/L=4.0��10-20mol/L��
�ʴ�Ϊ��4.0��10-20mol/L��
��3����������������Һ���ܹ�����ˮ�������������������Լ��������ữ�������������ӵ�ˮ�⣬
�ʴ�Ϊ������Al3+ˮ��
��4����������ͼ����ᾧ���ⲽ�����У�ĸҺ��������Ũ������ȴ�ᾧ������ϴ�ӵõ����壻
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
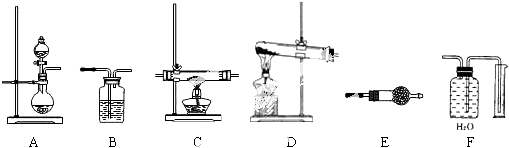
��5����������ͼ������롱�������Ӧ�������ǣ�ͨ�����ˣ����������������Һ�з��룬ϴ�Ӹ���õ�������CD��ȷ��
��ѡCD��
��6��a����ȡ0.906g�������Ʒ���������գ���Al2O3��������0.1020g�������������ʵ���Ϊ��
=0.001mol������0.002mol�����ӣ�0.4530g��Ʒ�к���0.001mol�����ӣ�����Ϊ0.027g
b����ȡ0.4530g�������Ʒ������������ˮ�ܽ⣬�ټ����Թ�����BaCl2��Һ�����»������ˡ��һ�����BaSO40.4659g�����ᱵ�����ʵ���Ϊ��
=0.002mol��������������ӵ����ʵ���Ϊ0.002mol������Ϊ0.192g��
c����ȡ0.4530g��Ʒ������������NaOH��Һ�����ȣ�������������ͨ����ʯ�Һ�Ũ���ᣬŨ��������0.0170g��Ũ�������ص�����Ϊ�������������������ʵ���Ϊ��
=0.001mol������ӵ����ʵ���Ϊ0.001mol������Ϊ0.018g��
0.4530g��Ʒ�У�����ˮ������Ϊ��0.4530-0.027-0.192-0.018=0.2160g��ˮ�����ʵ���Ϊ��
=0.012mol��
n��NH4+����n��Al3+����n��SO42-������n��H2O��=0.001��0.001��0.012��0.012=1��1��2��12��
����������Ļ�ѧʽ��NH4Al��SO4��2?12H2O
�ʴ�Ϊ��NH4Al��SO4��2?12H2O��
�ʴ�Ϊ��ȡ������Һ���Թ��У��ữ�����μӼ�������KMnO4��Һ��������������˵��Fe2+�ѳ�������ȡ�������Ӻ����Һ��������H2O2��Һ�����μ�KSCN��Һ��������������˵��Fe2+�ѳ�����
��2��KSP[Fe��OH��3]=4.0��10-38��PH=8����Һ������������Ũ��Ϊ��1��10-6mol/L��c��Fe3+��=
| 4.0��10-38 |
| (10-6)3 |
�ʴ�Ϊ��4.0��10-20mol/L��
��3����������������Һ���ܹ�����ˮ�������������������Լ��������ữ�������������ӵ�ˮ�⣬
�ʴ�Ϊ������Al3+ˮ��
��4����������ͼ����ᾧ���ⲽ�����У�ĸҺ��������Ũ������ȴ�ᾧ������ϴ�ӵõ����壻
�ʴ�Ϊ������Ũ������ȴ�ᾧ��
��5����������ͼ������롱�������Ӧ�������ǣ�ͨ�����ˣ����������������Һ�з��룬ϴ�Ӹ���õ�������CD��ȷ��
��ѡCD��
��6��a����ȡ0.906g�������Ʒ���������գ���Al2O3��������0.1020g�������������ʵ���Ϊ��
| 0.1020g |
| 102g/mol |
b����ȡ0.4530g�������Ʒ������������ˮ�ܽ⣬�ټ����Թ�����BaCl2��Һ�����»������ˡ��һ�����BaSO40.4659g�����ᱵ�����ʵ���Ϊ��
| 0.4659g |
| 233g/mol |
c����ȡ0.4530g��Ʒ������������NaOH��Һ�����ȣ�������������ͨ����ʯ�Һ�Ũ���ᣬŨ��������0.0170g��Ũ�������ص�����Ϊ�������������������ʵ���Ϊ��
| 0.0170g |
| 17g/mol |
0.4530g��Ʒ�У�����ˮ������Ϊ��0.4530-0.027-0.192-0.018=0.2160g��ˮ�����ʵ���Ϊ��
| 0.2160g |
| 18g/mol |
n��NH4+����n��Al3+����n��SO42-������n��H2O��=0.001��0.001��0.012��0.012=1��1��2��12��
����������Ļ�ѧʽ��NH4Al��SO4��2?12H2O
�ʴ�Ϊ��NH4Al��SO4��2?12H2O��
���������⿼�鹤�����̡����Ӽ��顢����ˮ�⡢�������Ŀ���ѡ�����ʵķ����ᴿ�ȣ����������ԭ���ǽ���Ĺؼ����Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ��������������������Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
�����йص�������ȷ���ǣ�������
A�� 2��3���� |
B�� 3�һ�1��ϩ |
| C����CH3CH2��2CHCH3��3������ |
D�� ������ |





 ʱ��A�����ķ�Ӧ����Ϊ
ʱ��A�����ķ�Ӧ����Ϊ