��Ŀ����
��8�֣�ij���������Al��(NH4)2SO4��MgCl2��AlCl3��FeCl2�е�һ�ֻ�����ɣ���ȡ�ù����������ʵ�飬����������й�����������ʾ(������������ݾ�������ɱ�״����)��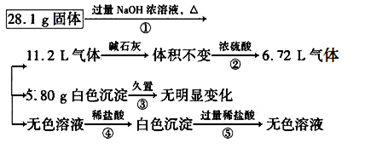
��ش��������⣺
��1����������Ƿ����FeCl2��________(����ڡ������ڡ�)��
��2����������Ƿ����(NH4)2SO4��______(����ڡ������ڡ�)������ж�������____________��
��3��д����Ӧ�ܵ����ӷ���ʽ��____________________��
��4������ݼ����жϻ�������Ƿ����AlCl3��_____(д������ж����ݣ�������д�������)��
��1�������ڡ�
��2�����ڡ� 11.2 L����ͨ��ŨH2SO4ʱ����������� 4.48 L��
��3��H����OH��===H2O��AlO2-��H����H2O===Al(OH)3����
��4��������У�MgCl2��Al��(NH4)2SO4���������պõ��� 28.1 g��˵������AlCl3
������������������������ͼ֪����������������������Һ��Ӧ���ɵ�����ͨ����ʯ��������䣬��ͨ��Ũ���������С��˵��ʣ��� 6.72 L����Ϊ��������ԭ������һ�����н���Al����������Ϊ5.4 g�����ɰ��������ʵ���Ϊ0.2 mol����ԭ������һ������ 0.1 mol ��NH4��2SO4��������Ϊ13.2 g���õ���ɫ�������ò���ɫ��˵����FeCl2�������������ױ�����Ϊ����ɫ����������������ΪNaOH����������ɫ���������ܺ���������������˵��5.8 g��ɫ����ΪMg��OH��2��Ϊ0.1 mol���������MgCl2Ϊ0.1 mol������Ϊ9.5g����ɫ��Һ����NaAlO2�������������ơ���1����ɫ�������ò���ɫ��˵��������FeCl2�������������ױ�����Ϊ����ɫ����������������2������ͨ��Ũ������������4.48 L��˵����������NH3����ԭ������һ�����ڣ�NH4��2SO4����3����ɫ��Һ������ɫ��Һ����NaAlO2�������������ƣ��������ᷢ����Ӧ�����ӷ���ʽΪH����OH��===H2O��AlO2-��H����H2O===Al(OH)3������4������������֪���������һ������Al����NH4��2SO4��MgCl2�������ʣ���m��Al��+m����NH4��2SO4��+m��MgCl2��="28.1" g������һ��û��AlCl3
���㣺���������ƶϡ�

�������ʵı��淽����ȷ���ǣ�
| A������������Һ��ʢ���ڴ���Ƥ���IJ���ƿ�� |
| B������������Һ��ʢ���ڴ��������IJ���ƿ�� |
| C��������������ˮ�� |
| D���������ƿ��Գ���¶���ڿ����д�� |
����ʵ�鷽���У����ܲⶨNa2CO3��NaHCO3,�������Na2CO3������������
| A��ȡa�˻����������ϡ�����ַ�Ӧ���ݳ������ü�ʯ�����գ�����b�� |
| B��ȡa�˻����������ϡ�����ַ�Ӧ�����ȡ����ɡ����գ���b�˹��� |
| C��ȡa�˻�����ּ��ȣ���b�˹��� |
| D��ȡa�˻����������Ca(OH)2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ���ɣ���b�˹��� |
��0.2mol ����CO2��ȫ��Ӧ�����ù��������Ϊ( )
����CO2��ȫ��Ӧ�����ù��������Ϊ( )
| A��21.2g | B��21.6g | C��22.0g | D��22.4g |
��ҵ���ú�����������ͭ�ķ����Ͻ�Ϊ��Ҫԭ����ȡ��������Һ������ͭ�������ˮ�Ȼ�������������������ͼ��ʾ��
��֪�������ʵ�PH��Χ��ʹFe3+������ȫ��Cu2+���������
��ش��������⣺
��1��д����Ͻ��м���KOH��Һ����������Ӧ�����ӷ���ʽ�� ��
��2������ҺA��ֱ�Ӽ�����������õ�����������Һ�г������������⣬��һ�����е�������
(�ѧʽ)��
�����һ����������ʵ�鷽������ҺA�Ʊ���������������Һ��������ͼ��ʽ�����Ʊ�����ͼ(��ʾ���ڼ�ͷ���·���������Լ���ʵ�����) ��
��3�� д���Լ�X������ ��
��4�� ʵ�����I��˳������Ϊ (ѡ�����)��
| A������ | B������Ũ�� | C������ | D����ȴ�ᾧ |
��6��д������E��W��Ӧ�Ļ�ѧ����ʽ�� ��
�Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȡ�ʵ����ģ�ҵ���������Ʊ����죨Fe2O3�����������£�
��1���������ijɷ�������������������� ��д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ ��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ�� ������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
��3������A����Ҫ�ɷ�Ϊ ����ҺB���Ի��յ�������____________��
��4������ϴ�ӹ��̵�ʵ����� ��
��5����֪����������Ϊw kg�����������Ʊ������У���Ԫ�����25%�����յõ����������Ϊm kg����ԭ������������Ԫ����������Ϊ ��������������ʽ��ʾ����

