��Ŀ����
10�������£����ᡢ������볣����Ka��HCOOH��=1.77x10-4��Ka��CH3COOH��=1.75��10-5�мס��ҡ���������Һ��| �� | 1000mL 0.1mol/L HCOONa��Һ |
| �� | 1000mL 0.1mol/L CH3COONa��Һ |
| �� | 1000mL��HCOONa��CH3COONa��0.05mol����Һ |
| A�� | ��Һ��c��Na+������=�ң��� | |
| B�� | ��Һ�����������Ӻ�������������ף������� | |
| C�� | ��ҺpH���ף������� | |
| D�� | ��Һ�������������������ף������� |
���� ���к����ʵ���Ũ�Ⱦ�Ϊ0.05mol/L��HCOONa��CH3COONa�����ɼ��ᡢ������볣����Ka��HCOOH��=1.77x10-4��Ka��CH3COOH��=1.75��10-5�����Լ��������ǿ�����ᣬ���Լ�������ӵ�ˮ��̶�С����������ӣ��ɴ˷������
��� �⣺A��������Һ��c��Na+��=0.1mol/L��������Һ��c��Na+������=�ң�������A����
B��ˮ��̶�Խ����������������ӵ���Ŀ�ͷ�����ĿԽ�࣬����������Ŀ��ȣ�������Һ�����������Ӻ�������������ף������ң���B��ȷ��
C��ˮ��̶�Խ��pHԽ�����������ˮ��̶�������ߵĻ�����֮���������Ǽ�������ӣ�������ҺpH���ף������ң���C��ȷ��
D�����ݵ���غ����Һ������������������������������2�����������ӵ���Ŀ��ͬ��ˮ��̶�Խ�������ӵ���ĿԽС��ˮ��̶�ԽС�������ӵ���ĿԽ�࣬������Һ�������������������ף������ң���D��ȷ��
��ѡA��
���� ���⿼��������ϵĶ����жϼ���Һ����Ũ�ȴ�С�ıȽϣ���Ŀ�Ѷ��еȣ�ע������������Һ���������ҺpH�Ĺ�ϵ���ܹ����õ���غ㡢�����غ㼰�ε�ˮ��ԭ���ж�����Ũ�ȴ�С������������ѧ�������Ӧ��������

 ��У����ϵ�д�
��У����ϵ�д�| A�� | �ȶ��ԣ�NH3��PH3��SiH4 | B�� | ���ԣ�HClO4��H2SO4��H3PO4 | ||
| C�� | ���ԣ�KOH��NaOH��Mg��OH��2 | D�� | �����ԣ�F2��Cl2��Br2 |
| A�� | ��̪�����к������ֹ����� | |
| B�� | ��̪�ķ���ʽΪC20H12O4 | |
| C�� | ��̪���ڷ����� | |
| D�� | ��̪�ṹ�к����ǻ���-OH�����ʷ�̪���ڴ� |
| A�� |  ͼ��֤FeCl3��H2O2�ֽⷴӦ�д����� | |
| B�� |  ͼ�����к��ȵIJⶨ | |
| C�� |  ͼ����̽��Cu��������ԭ���ԭ�� | |
| D�� |  ͼ���ڱȽ����ᡢ̼�ᡢ��������� |
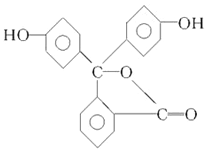
 ������X��һ��ҽҩ�м��壬��ṹ��ʽ��ͼ��ʾ�������йػ�����X��˵����ȷ���ǣ�������
������X��һ��ҽҩ�м��壬��ṹ��ʽ��ͼ��ʾ�������йػ�����X��˵����ȷ���ǣ�������| A�� | 1 mol X��һ�������������8 mol H2�ӳ� | |
| B�� | 1 mol X��һ���������������2 mol NaOH��Ӧ | |
| C�� | X���ж��ֺ��������ţ�����������ˮ�������Ҳ�ж��ֺ��������� | |
| D�� | X����ʽΪC16H12O4�����Ҵ�������Ӧ���ɷ���ʽΪC18H18O5���� |
| A�� |  �ռ�NO2����ֹ����Ⱦ���� | |
| B�� |  ��ȡһ������NaOH | |
| C�� |  ����Ũ������ͭ��Ӧ��IJ����У��Ƿ���ͭ���� | |
| D�� |  ��ȡNaHCO3 |

 G��
G��
 ����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���ԭ���������ٵ�һ�ֽṹ��ʽΪ��
����������ȼ��Ʒ�ʿ������ܵIJ��������A��ͬ���칹���к���ԭ���������ٵ�һ�ֽṹ��ʽΪ�� ����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ���д����ϩ�������п��ܵĽṹ��ʽ
����A����ϩ����H2ͨ���ӳɷ�Ӧ�õ���д����ϩ�������п��ܵĽṹ��ʽ ��
�� ��
��