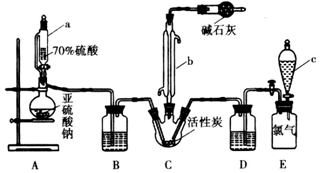
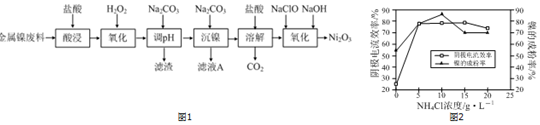
��Ŀ����
2���������ƣ�NaClO2����һ����Ҫ��ɱ����������Ҳ������Ư��֯��ȣ�����������NaClO2•3H2O�ֲ�Ʒ�Ĺ�������ͼ1��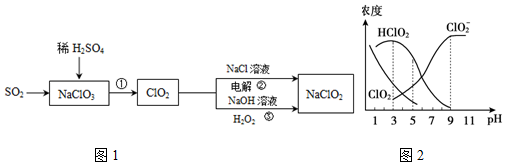
��1��NaClO2��Cl�Ļ��ϼ���+3���ݻ��ϼ��Ʋ�NaClO2���������ԡ���ԭ���ԣ�
��2����Ӧ�������ӷ���ʽ��2ClO3-+SO2�TSO42-+2ClO2����2ClO3-+SO2+2H+�T2HSO4-+2ClO2���� ��״���£�ÿ����22.4 L ClO2���壬����SO232g��
��3����Ӧ�ڡ���⡱�У���������������ͨ��������������������������һ�����������
��4����Ӧ�۷�����Ӧ�Ļ�ѧ����ʽ��2ClO2+H2O2+2NaOH�T2NaClO2+2H2O+O2���÷�Ӧ�л�ԭ���������������ʵ���֮��Ϊ1��2��
��5���������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ2��ʾ��Cl-û�л�����������˵����ȷ����ad������ĸ����
a�����������ڼ��������½��ȶ�
b��NaClO2��Һ�У�$\frac{c��N{a}^{+}��}{c��Cl{O}_{2}^{-}��}$=1
c��ʹ�ø�Ư�������pHΪ3
d����NaClO2��Һ�е���ϡ���ᣬ��pH��6ʱ����Һ����Ҫ��Ӧ�ǣ�ClO2-+H+�THClO2��
���� ��1�����ݻ������л��ϼ۴�����Ϊ0�ɵã�Ԫ�ش����м��̬�������Ժͻ�ԭ�ԣ�
��2����Ӧ�������������������ԣ����������л�ԭ�ԣ������Ի���������ClO2����������ӣ����ݷ�Ӧ��Ԫ�ص�ʧ��������ȿɵã�
��3����⡱�У���������������ͨ���õ��ӻ������������õ���һ��Ϊ������
��4����Ӧ�۷�����ӦΪClO2��H2O2���������������������ƺ������ķ�Ӧ����ԭ��Ϊ�������⣬������ΪClO2��
��5��a����ͼ���Եó�������������ClO2-Ũ�ȸߣ�
b��NaClO2��Һ��������ˮ�⣻
c���������⣺HClO2��ClO2������Ư�����ã����ͼ��HClO2��ClO2��Ũ�ȴ�С��ȷ��ʹ�ø�Ư�������pH��
d��pH��6ʱ����ͼ��֪����Һ����Ҫ��ClO2-��
��� �⣺��1���������л��ϼ۴�����Ϊ0��NaClO2����Ԫ��+1�ۣ���Ԫ��-2�ۣ�����Ԫ��+3�ۣ���NaClO2���������ԡ���ԭ�ԣ�
�ʴ�Ϊ��+3�������ԡ���ԭ�ԣ�
��2����Ӧ�������ӷ���ʽ�ǣ�2ClO3-+SO2�TSO42-+2ClO2����2ClO3-+SO2+2H+�T2HSO4-+2ClO2������״���£�22.4 L ClO2���弴Ϊ1mol����Ӧ��ClԪ�ػ��ϼ���+5�۽��͵�+4�ۣ���ת��1mol���ӣ�����Ԫ�ػ��ϼ۴�+4�����ߵ�+6�ۣ���SO2��0.5mol������Ϊ0.5mol��64g/mol=32g��
�ʴ�Ϊ��2ClO3-+SO2�TSO42-+2ClO2����2ClO3-+SO2+2H+�T2HSO4-+2ClO2����32g��
��3����⡱�У���������������ͨ���õ��ӻ������������õ���һ��Ϊ������
�ʴ�Ϊ��������
��4����Ӧ�۷�����ӦΪ2ClO2+H2O2+2NaOH�T2NaClO2+2H2O+O2��ClO2����Ԫ�ػ��ϼ۽��͵õ�������������H2O2����Ԫ�ػ��ϼ�����ʧ��������ԭ�����÷�Ӧ�л�ԭ���������������ʵ���֮��Ϊ��1��2��
�ʴ�Ϊ��2ClO2+H2O2+2NaOH�T2NaClO2+2H2O+O2��1��2��
��5��a����ͼ���Եó�������������ClO2-Ũ�ȸߣ����ڼ����������������ƽ��ȶ�����a��ȷ��
b��NaClO2��Һ��������ˮ�⣬��$\frac{c��N{a}^{+}��}{c��Cl{{O}_{2}}^{-}��}$��1����b����
c��HClO2��ClO2������Ư�����ã����ͼ��HClO2��ClO2��Ũ��Խ��Ϊʹ�ø�Ư�������pH��Ӧ����4-5����c����
d��pH��6ʱ����ͼ��֪����Һ����Ҫ��ClO2-������NaClO2��Һ�е���ϡ���ᣬ��Һ����Ҫ��Ӧ�����ӷ���ʽ�ǣ�ClO2-+H+�THClO2����d��ȷ��
�ʴ�Ϊ��a d��
���� ���⿼�����ʵ��Ʊ���Ϊ��Ƶ���㣬�漰ѧ���ķ������������������Ŀ��飬����������ԭ��Ӧ�ǹؼ���ͬʱע�⼰����ˮ��ȣ���Ŀ�Ѷ��еȣ�

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | 1 mol?L-1 A1C13��Һ�к��е�Al3+��ĿС��NA | |
| B�� | ��״���£�11 g3H216O�к��е�������ĿΪ6NA | |
| C�� | 1 mol Li2O��Na2O2�Ļ�����к��е�������������3NA | |
| D�� | ���³�ѹ�£�4.6 g NO2�����ĵ�ԭ����ĿΪ0.1NA |
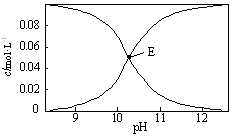 20��ʱ������һ��c��Na2CO3��+c��NaHCO3��=0.100mol•L-1�Ļ����Һ����Һ��c��HCO3-����c��CO32-����pH�Ĺ�ϵ��ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
20��ʱ������һ��c��Na2CO3��+c��NaHCO3��=0.100mol•L-1�Ļ����Һ����Һ��c��HCO3-����c��CO32-����pH�Ĺ�ϵ��ͼ��ʾ������ָ����Һ���������ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | pH=9����Һ�У�c��HCO3-����c��H2CO3����c��CO32-�� | |
| B�� | c��HCO3-��=c��CO32-����E����Һ�У�c��OH-��+c��CO32-����c��H+��+c��H2CO3��+0.050 mol•L-1 | |
| C�� | pH=11����Һ�У�c��Na+����2c��CO32-��+c��HCO3-�� | |
| D�� | 0.100 mol•L-1��Na2CO3��Һ�У�c��H+��+c��H2CO3��+c��HCO3-��=c��OH-�� |
| A�� | ������������ʵ���Ũ�ȵ�NaX������HX��Ϻ����ҺpH��7����һ���У�c��Na+����c��HX����c��X-����c��H+����c��OH-�� | |
| B�� | 1L0.1mol•L-1CuSO4•��NH4��2SO4•6H2O����Һ�У�c��SO42-����c��NH4+����c��Cu2+����c��H+����c��OH-�� | |
| C�� | 0.1mol•L-1NaHCO3��Һ�У�c��Na+��+c��H+��+c��H2CO3����c��HCO3-��+c��CO32-��+c��OH-�� | |
| D�� | ���ʵ���Ũ�ȷֱ�Ϊc1��c2�����ִ�����Һ������pH�ֱ�Ϊa��a+1����c1��10c2 |
| ʵ�鲽�� | ʵ������ |
| a��ȡ��������Һ���Ӽ��μ��� | ��Һ���ɫ |
| b��ȡ��������Һ����Ũ������CuƬ��ŨH2SO4������ | ����ɫ����������������������Ա�ɺ���ɫ |
| c��ȡ��������Һ����BaCl2��Һ | �а�ɫ�������� |
| d��ȡ��������Һ����AgNO3��Һ | ���ȶ��İ�ɫ�������ɣ��Ҳ�����HNO3 |
| e��ȡ��������Һ����NaOH��Һ | �а�ɫ�������ɣ���NaOH����ʱ���������ܽ� |
��1����Һ�п϶����ڵ������У�NO3-��SO42-��Mg2+��Al3+��Cl-��Һ�п϶������ڵ������У�I-��Ba2+��Fe2+��HCO3-�������һ��ʵ�����ȷ���Ƿ����K+�� �������ӷ��ţ�
��2����д������b����Һ�������������ӷ���ʽ��3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O��
| A�� | c��OH-����c��H+����c��NH4+����c��SO42-�� | B�� | c��OH-����c��NH4+����c��SO42-����c��H+�� | ||
| C�� | c��SO42-��+c��OH-����c��NH4+��+c��H+�� | D�� | c��SO42-��+c��OH-��=c��NH4+��+c��H+�� |
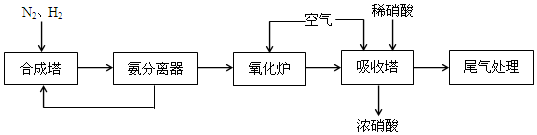
 2NH3���÷�Ӧ�е���������N2��
2NH3���÷�Ӧ�е���������N2��