��Ŀ����
 ��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨ʵ��1��CO2�����Ϊ��״���£�����������ͬ��ͬѹ�²ⶨ����
��ͼ��ʾ�����ס�������װ�в�ͬ���ʵ���Ͳ�õ�������������������Ͳ�ڵ�����ѹ������Ͳ�ڣ������±����еIJ�ͬʵ�飨ʵ��1��CO2�����Ϊ��״���£�����������ͬ��ͬѹ�²ⶨ���� | ʵ����� | ����Ͳ������ | ����Ͳ������ | ����Ͳ������ |
| 1 | 10mL 0.1mol/LNaOH��Һ | 22.4mLCO2 | ���������� |
| 2 | 20mL H2S | 10mL SO2 | |
| 3 | 30mL NO2 | 10mL H2O��l�� | ʣ����ɫ���壬�����Զ�����ѹ�� |
| 4 | 15mL Cl2 | 40mL NH3 |
��1��ʵ��1�У���Ӧ�Ļ�ѧ����ʽΪ
��2��ʵ��2����Ͳ�ڵ������ǣ���
��3��ʵ��3�У��������ʣ�����ɫ����������
��4��ʵ��4�У���֪��3Cl2+2NH3�TN2+6HCl������Ͳ���������ƶ�����Ͳ���а��̲����⣬�������ɫ�ɻ���ɫ��Ϊ��ɫ�������Ͳ��ʣ����������ԼΪ
���㣺��ѧƽ�⽨���Ĺ���
ר�⣺��ѧƽ��ר��
��������1������NaOH��CO2���ʵ��������ʵ���������ȷ����Ӧ�Ļ�ѧ����ʽ��
��2������H2S��SO2��Ӧ���ɵ������ˮ������ѹǿ�仯��
��3������N2O4��ת��ΪNO2���Լ�NO2��ˮ��Ӧ���������һ��������������
��4����������ɫΪ����ɫ��3Cl2+2NH3�TN2+6HCl������İ���������Ȼ��ⷴӦ������
��2������H2S��SO2��Ӧ���ɵ������ˮ������ѹǿ�仯��
��3������N2O4��ת��ΪNO2���Լ�NO2��ˮ��Ӧ���������һ��������������
��4����������ɫΪ����ɫ��3Cl2+2NH3�TN2+6HCl������İ���������Ȼ��ⷴӦ������
���
�⣺��1��NaOH��CO2���ʵ����ֱ�Ϊ��0.001mol��0.001mol�����ʵ�������Ϊ1��1��ȷ����Ӧ�Ļ�ѧ����ʽ��NaOH+CO2=NaHCO3���ʴ�Ϊ��NaOH+CO2=NaHCO3��
��2��20mLH2S��10mLSO2��Ӧ2H2S+SO2=3S+2H2O����Ӧ������ɫ�������Һ̬ˮ�����������С����Ͳ��ѹǿ��С�������Զ������ƶ���
�ʴ�Ϊ������ɫ���壻���ڣ�
��3��������30mL������NO2��N2O4�Ļ�����壬��Ҫ�Ƕ����������壬����10mLH2O��l������3NO2+H2O=2HNO3+NO��֪����10mLNO���������ʣ�����ɫ������һ��������NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO��
�ʴ�Ϊ��10��3NO2+H2O=2HNO3+NO��
��4��15molCl2��40mLNH3����Ϸ�����Ӧ
���ݷ���ʽ��֪��3Cl2+2NH3 �TN2 +6HCl��
3 2 1 6
15mol 10mol 5mol 30mol
ʣ�ఱ�����ʵ���Ϊ40mol-10mol=30mol��
�� NH3+HCl�TNH4Cl
30mol 30mol
��֪�����ɵ�HCl��ʣ���NH3��Ӧ����NH4Cl���Ȼ���Ͱ���ǡ�÷�Ӧ��Cl2����������ȫ��Ӧ���������ʣ�������Ϊ���������ʵ���Ϊ5mol��
�ʴ�Ϊ��5��
��2��20mLH2S��10mLSO2��Ӧ2H2S+SO2=3S+2H2O����Ӧ������ɫ�������Һ̬ˮ�����������С����Ͳ��ѹǿ��С�������Զ������ƶ���
�ʴ�Ϊ������ɫ���壻���ڣ�
��3��������30mL������NO2��N2O4�Ļ�����壬��Ҫ�Ƕ����������壬����10mLH2O��l������3NO2+H2O=2HNO3+NO��֪����10mLNO���������ʣ�����ɫ������һ��������NO2��H2O��Ӧ�Ļ�ѧ����ʽΪ��3NO2+H2O=2HNO3+NO��
�ʴ�Ϊ��10��3NO2+H2O=2HNO3+NO��
��4��15molCl2��40mLNH3����Ϸ�����Ӧ
���ݷ���ʽ��֪��3Cl2+2NH3 �TN2 +6HCl��
3 2 1 6
15mol 10mol 5mol 30mol
ʣ�ఱ�����ʵ���Ϊ40mol-10mol=30mol��
�� NH3+HCl�TNH4Cl
30mol 30mol
��֪�����ɵ�HCl��ʣ���NH3��Ӧ����NH4Cl���Ȼ���Ͱ���ǡ�÷�Ӧ��Cl2����������ȫ��Ӧ���������ʣ�������Ϊ���������ʵ���Ϊ5mol��
�ʴ�Ϊ��5��
���������⿼����Ԫ�ؼ��仯�����֪ʶ���漰�����ȡ���Ļ���������ʣ��ۺ��Խ�ǿ����ѧϰ�ý�֪ʶʱ������صķ�Ӧ����ʽ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
 һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯����������ͼ��ʾ������������ȷ���ǣ�������
һ���¶��£���2L���ܱ������У�X��Y��Z������������ʵ�����ʱ��仯����������ͼ��ʾ������������ȷ���ǣ�������| A����Ӧ��ʼ��10s����Z��ʾ�ķ�Ӧ����Ϊ0.158mol/��L?s�� |
| B����Ӧ��ʼ��10s��X�����ʵ���Ũ�ȼ�����0.79mol/L |
| C����ƽ��ʱY�����ʵ�������Ϊ��9.5% |
| D����Ӧ�Ļ�ѧ����ʽΪ��X��g��+Y��g��?Z��g�� |
���ڵ���Ȼ�����Һ������������ȷ���ǣ�������
| A�����ʱ�������õ��������������õ������� |
| B�������������л���ɫ��������� |
| C����������������Һ�е����̪��Һ����Һ����ɫ |
| D�����һ��ʱ������Һȫ��ת�Ƶ��ձ��У���ֽ������Һ������ |
0.1mol/LHF��Һ��pH=2�������Һ���й�Ũ�ȹ�ϵʽ����ȷ���ǣ�������
| A��c��H+����c��F-�� |
| B��c��H+����c��HF�� |
| C��c��OH-����c��HF�� |
| D��c��HF����c��F-�� |
������ʵ��װ����ɶ�Ӧ��ʵ�飬���ܴﵽʵ��Ŀ���ǣ�������
| A | B | C | D | |
| װ�� | 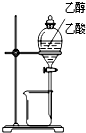 | 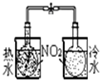 | 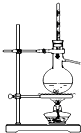 |  |
| ʵ�� | �����Ҵ������� | ֤���¶ȶԻ�ѧƽ���Ӱ�� | ����е����ϴ�Ļ���Һ������ | ��ȡ���ռ����� |
| A��A | B��B | C��C | D��D |
 ���ڴﵽƽ��Ŀ��淴Ӧ��X+Y?W+Z������ѹǿ�������淴Ӧ���ʣ�v���ı仯��ͼ��ʾ��������֪X��Y��Z��W�ľۼ�״̬�����ǣ�������
���ڴﵽƽ��Ŀ��淴Ӧ��X+Y?W+Z������ѹǿ�������淴Ӧ���ʣ�v���ı仯��ͼ��ʾ��������֪X��Y��Z��W�ľۼ�״̬�����ǣ�������| A��X��Y��Z��W��Ϊ���� |
| B��Z��WΪ���壬X��Y��֮һΪ���� |
| C��X��Y��Z��Ϊ���壬WΪ������ |
| D��X��YΪ���壬Z��W��������һ��Ϊ���� |
 ���ܱ������м����Ũ�ȵ�CO��H2O��T��ʱ�������·�Ӧ��
���ܱ������м����Ũ�ȵ�CO��H2O��T��ʱ�������·�Ӧ��