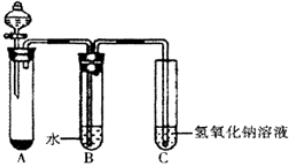
��Ŀ����
5��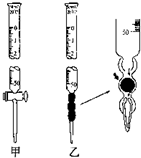 ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ
ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ���õζ��ķ������ⶨ�����Ũ�ȣ�ʵ�����������ʾ��
| ʵ���� | ����HCl��Һ�����/mL | ����NaOH��Һ�����/mL |
| 1 | 20.00 | 22.00 |
| 2 | 20.00 | 22.10 |
| 3 | 20.00 | 21.90 |
�ڵζ�����ͼ��ʾ�����ҵζ���ʢװ��Ũ�ȵ�����������Һ ����ס����ҡ�����
�����в�����ʹ����õ������Ũ��ƫ�͵���D��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ�����
���� �ټ���ʵ���������������������Һ��ƽ������������Ӧ������ϵ���㣬�����к͵ζ�ԭ�����������Ũ�ȣ�
������������ҺΪ����Һ�������Һ�������ѡ��ζ��ܣ�
�۸���c�����⣩=$\frac{c������V������}{V�����⣩}$��������������V����������Ӱ�죬�Դ��ж�Ũ�ȵ���
��� �⣺��ͼ���е�ʵ�����ݼ�������������Һ���ĵ������V��NaOH��=$\frac{22.00ml+22.10ml+21.90ml}{3}$=22ml��H++OH-=H2O��
��δ֪�����Ũ��=$\frac{0.220L��0.80mol/L}{0.020L}$=0.88 mol•L-1��
�ʴ�Ϊ��0.88 mol•L-1��
��NaOH��Һ�Լ��ԣ���Ӧ�ü�ʽ�ζ���ʢװ��Ũ�ȵ�����������Һ���ʴ�Ϊ���ң���
��A��ʢװ����Һ����ƿ��ˮϴ��δ����Բⶨ����أ���A����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ����ȡ����Һ������������V������ƫ����c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��HCl��ƫ�ⶨ���ƫ��B����
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ���ζ����������ĵı���Һ���������c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��HCl��ƫ�ⶨ���ƫ��C����
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ�������ȡ����Һ�����С������c�����⣩=$\frac{c������V������}{V�����⣩}$��֪���ⶨc��HCl��ƫ�ͣ��ⶨ���ƫ�ͣ���D��ȷ��
�ʴ�Ϊ��D��
���� ������Ҫ�������к͵ζ��������������Լ����㣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ������к͵ζ���ԭ���ǽ���ؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | SO2ʹ��ˮ��ɫ����ϩʹKMnO4��Һ��ɫ��ԭ����ͬ | |
| B�� | Ũ��ˮ�еμ�FeCl3������Һ���Ƶ�Fe��OH��3���� | |
| C�� | �轺������װʳƷ�ĸ���� | |
| D�� | ���������������뵼����������۵��Ӳ�ȴ��� |
| A�� | ��ʯ�Ҷ�úȼ�պ��γɵ�������������ʯ����Ͻ��ܼ��ŵ����� | |
| B�� | �Է���ӦҲ�������ؼ�С�ķ�Ӧ | |
| C�� | ���ε�طŵ�����еķ�Ӧ�����Է���Ӧ | |
| D�� | ��C2H2��ȼ����Ϊ1300 kJ•mol-1������ȼ�չ�����ÿת��5NA�����ӻ�ų�1300 kJ������ |
| A�� | ʹ�ô����ܹ����ͻ�ѧ��Ӧ�ķ�Ӧ�ȡ�H | |
| B�� | HCl��NaOH��Ӧ���к��ȡ�H=-57.3 kJ/mol����H2SO4��NH3•H2O��Ӧ���к��ȡ�H=2����-57.3��kJ/mol | |
| C�� | ��Ҫ���Ȳ��ܷ����ķ�Ӧһ�������ȷ�Ӧ | |
| D�� | CO��g����ȼ������283.0 kJ/mol����2CO2��g��=2CO��g��+O2��g���ġ�H=+2��283.0 kJ/mol |
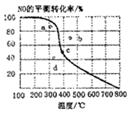 ��ͼ������Ϊһ��ѹǿ��NO��ƽ��ת�������¶ȵĹ�ϵ��ͼ��a��b��c��d�ĵ��ʾ��ͬ�¶ȡ�ѹǿ��2NO��g��+O2��g��?2NO2��g���ﵽƽ��ʱNO��ת���ʣ���ѹǿ���ĵ�Ϊ��������
��ͼ������Ϊһ��ѹǿ��NO��ƽ��ת�������¶ȵĹ�ϵ��ͼ��a��b��c��d�ĵ��ʾ��ͬ�¶ȡ�ѹǿ��2NO��g��+O2��g��?2NO2��g���ﵽƽ��ʱNO��ת���ʣ���ѹǿ���ĵ�Ϊ��������| A�� | a | B�� | b | C�� | c | D�� | d |
��1���ζ�Ӧ��ѡ�÷�̪��ָʾ��
��2�����¶���CH3COOH�ĵ��볣��Ka=2��10-5��
��3�������ʵ��Ũ��Ϊ0.1006mol/L��������λ��Ч���֣�
��4�����������������ⶨ���ƫ�ߵ���AC��
A����ʽ�ζ���δ�ñ���Һ��ϴ
B����ƿδ�ô���Һ��ϴ
C���ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D���ζ�ǰ���ζ����е���ҺҺ����͵��ڡ�0��������
��5���ζ��ķ���������к͵ζ��������ζ�����ϵζ���������ԭ�ζ��ȣ������ζ����õ�ָʾ����������һ�ֳ���������֪һЩ���ε���ɫ��Ksp��20�棩���£��ⶨˮ�����Ȼ���ĺ��������ñ���������Һ���еζ���
| ��ѧʽ | AgCl | AgBr | AgI | Ag2S | Ag2CrO4 |
| ��ɫ | ��ɫ | dz��ɫ | ��ɫ | ��ɫ | ��ɫ |
| Ksp | 1.8��10-10 | 5.0��10-13 | 8.3��10-17 | 2.0��10-48 | 1.8��10-10 |
A��KBr B��KI C��K2S D��K2CrO4
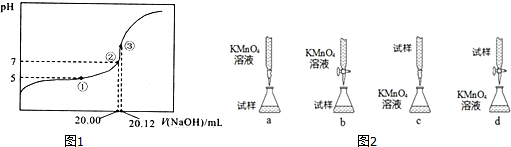
��6�������������£���KMnO4����Һ����������ԭ�ζ������ԲⶨFe2+�ĺ�������Ӧ�����ӷ���ʽ�ǣ�Fe2++MnO4-+H+--Fe3++Mn2++H2O��δ��ƽ��ͼ2���ֵζ���ʽ���гֲ�����ȥ��������Ϊ���������b������ĸ��ţ����жϵζ��յ�����ݵ������һ��KMnO4��Һǡ�����ػ�ɫ����ɫ���Ұ�����ڲ���ɫ��
����˵���д�����ǣ�������
| A�� | KClO3�ڷ�Ӧ�еõ����� | |
| B�� | ClO2���ȵĻ��ϼ�Ϊ+4�� | |
| C�� | �ڷ�Ӧ��H2C2O4�ǻ�ԭ�� | |
| D�� | 1 mol KClO3�μӷ�Ӧ��2mol����ת�� |