��Ŀ����
17��������ԭ�ζ�ʵ��ͬ�к͵ζ����ƣ�����֪Ũ�ȵ���������Һ�ζ�δ֪Ũ�ȵĻ�ԭ����Һ��֮��������0.001mol•L-1����KMnO4��Һ��δ֪Ũ�ȵ���ɫNaHSO3��Һ����Ӧ�����ӷ���ʽ��2MnO4-+5HSO3-+H+�T2Mn2++5SO42-+3H2O��������������⣺
��1����ʵ�鲻��Ҫ�����Ҫ������Ҫ����ʹ��ָʾ����������Mn2+��ɫ��MnO4-Ϊ��ɫ��������MnO4-ʹ��ɫ��Һ��Ϊ��ɫ��
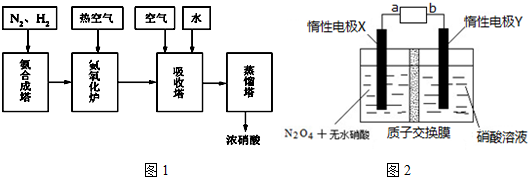
��2���õζ�ʵ�����������������е�A��D��E��F��G��H����
A����ʽ�ζ��ܣ�50mL����B����ʽ�ζ��ܣ�50ml�� C����Ͳ��10ml����D����ƿ�� E������̨�� F���ζ��ܼ� G���ձ��� H����ֽ�� I����ͷ�ιܡ� J��©��
��3�����ü��ᡱ���ʽ�ζ���ʢ������KMnO4��Һ���Է���ԭ��
����KMnO4��Һ����ǿ�����ԣ��ܸ�ʴ�ܣ�
��4���ζ�ǰƽ��KMnO4��ҺҺ�棬�̶�Ϊaml���ζ�����Һ��̶�Ϊbml����b-a��ml��ʵ������KMnO4��Һ����٣���ࡱ���١���������ζ�������Һ��̶�Ϊcml������õ��Ĵ���ҺŨ�ȱ�ʵ��Ũ�ȴ����С������
��5��ȡCe��OH��4��Ʒ0.5000g���������ܽ����0.100 0mol•L-1FeSO4����Һ�ζ����յ�ʱ���汻��ԭΪCe3+��������20.00mL����Һ���ò�Ʒ��Ce��OH��4����������Ϊ_83.20%��
���� ��1���������Ϊ��ɫ��Һ�������������Ʒ���������ԭ��Ӧ������ɫ�Ķ��������ӣ��ݴ˽��
��2�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫNaHSO3��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���Ҫ�ձ�����ƿ����ֽ���ζ��ܼк�����̨��
��3��������ؾ���ǿ�������ܸ�ʴ��ʽ�ζ����е��ܣ�
��4���ζ�����Һ�棬����ƫС���ζ�������Һ�棬����ƫ��c�����⣩=$\frac{c��������V������}{V�����⣩}$��$\frac{5}{2}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��5�����ݵ����غ㽨����ϵʽ��Ce��OH��4��FeSO4��Ȼ����м������Ce��OH��4������������������������
��� �⣺��1��Mn2+��ɫ��MnO4- Ϊ��ɫ��������MnO4-ʹ��ɫ��Һ��Ϊ��ɫ�����Ա�ʵ�鲻��Ҫʹ��ָʾ����
�ʴ�Ϊ������Ҫ����Mn2+��ɫ��MnO4- Ϊ��ɫ��������MnO4-ʹ��ɫ��Һ��Ϊ��ɫ��
��2�����Ը�����ؾ���ǿ�����ԣ�ʵ��ʱӦ����ʽ�ζ��ܣ���ɫNaHSO3��Һ�����ԣ�����ʽ�ζ��ܣ��ζ����̻���Ҫ��ƿʢ�Ŵ���Һ����ֽ�Ա��յ���ɫ�仯���ζ��ܼк�����̨��������Ҫ�õ����ǣ�A��D��E��F��G��H����
�ʴ�Ϊ��A��D��E��F��G��H����
��3��������ؾ���ǿ�������ܸ�ʴ��ʽ�ζ����е��ܣ����Բ����ü�ʽ�ζ���ʢ�Ÿ��������Һ��Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ�������KMnO4��Һ�ḯʴ��ʽ�ζ����¶˽��ܣ�
��4���ζ�ǰƽ��KMnO4Һ�棬�̶�Ϊa mL���ζ�����Һ��̶�Ϊb mL������ƫС����b-a��mL��ʵ������KMnO4��Һ����٣�
����ζ�������Һ��̶�Ϊcml����c-a��mL��ʵ�����ĸ���������������c�����⣩=$\frac{c��������V������}{V�����⣩}$��$\frac{5}{2}$��֪����ҺŨ�ȱ�ʵ��Ũ��ƫ��
�ʴ�Ϊ���٣� ��
��5��Ce��OH��4 ��FeSO4
0.0020mol 0.1000mol/L-1��0.020L
����m��Ce��OH��4��=0.0020mol��208g/mol=0.416g����Ʒ��Ce��OH��4����������Ϊ
$\frac{0.416g}{0.5g}$��100%=83.20%��
�ʴ�Ϊ��83.20%��
���� ���⿼���к͵ζ����������㣬Ϊ��Ƶ���㣬������ѧ���ķ����������ʵ�������Ŀ��飬ע������к͵ζ��IJ���ԭ����ʵ�鷽�����Ѷ��еȣ�

| A�� | BaSO4������ˮ������BaSO4�Ƿǵ���� | |
| B�� | ������ʵĵ�����һ����ǿ������� | |
| C�� | 25��ʱ0.1mol/L��CH3COOH��ҺpH=3��˵��CH3COOHΪ������� | |
| D�� | ij�����ܵ��磬���Ը�����һ���ǵ���� |
| ��� | ��������ĩ״����mol�� | ���Ũ�ȼ���� | ��Ӧ�¶ȣ��棩 | ||
| A | Al | 0.1 | 0.1mol•L-1 ���� | 10mL | 60 |
| B | Fe | 0.1 | 0.2mol•L-1���� | 10mL | 60 |
| C | Al | 0.1 | 18mol•L-1 ���� | 10mL | 60 |
| D | Mg | 0.1 | 0.2mol•L-1 ���� | 10mL | 60 |
| A�� | A | B�� | B | C�� | C | D�� | D |
 ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ
ijѧϰС����0.80mol/L��Ũ�ȵ��ռ���Һ�ⶨδ֪Ũ�ȵ����ᣮ���õζ��ķ������ⶨ�����Ũ�ȣ�ʵ�����������ʾ��
| ʵ���� | ����HCl��Һ�����/mL | ����NaOH��Һ�����/mL |
| 1 | 20.00 | 22.00 |
| 2 | 20.00 | 22.10 |
| 3 | 20.00 | 21.90 |
�ڵζ�����ͼ��ʾ�����ҵζ���ʢװ��Ũ�ȵ�����������Һ ����ס����ҡ�����
�����в�����ʹ����õ������Ũ��ƫ�͵���D��
A��ʢװ����Һ����ƿ��ˮϴ��δ����
B���ζ�ǰ����ʽ�ζ��ܼ�������ݣ��ζ���������ʧ
C����ʽ�ζ���������ˮϴ����δ�ñ�����������Һ��ϴ
D������ʽ�ζ��ܵĿ̶�ʱ���ζ�ǰ���Ӱ�Һ����ʹ����ζ����Ӷ�����
��1������100mL 0.10mol•L-1 NaOH����Һ������0.4g�������ƹ��壮
��2��ȡ20.00mL�������������ƿ�У����μ�2��3�η�̪��ָʾ�������Լ����Ƶ�NaOH����Һ���еζ����ظ������ζ�����2��3�Σ���¼�������£�
| ʵ���� | NaOH��Һ��Ũ�ȣ�mol•L-1�� | �ζ����ʱ��NaOH��Һ����������mL�� | ��������������mL�� |
| 1 | 0.10 | 22.62 | 20.00 |
| 2 | 0.10 | 22.72 | 20.00 |
| 3 | 0.10 | 22.80 | 20.00 |
�ڸ����������ݣ��ɼ�����������Ũ��ԼΪ0.11mol•L-1��������λ��Ч���֣���
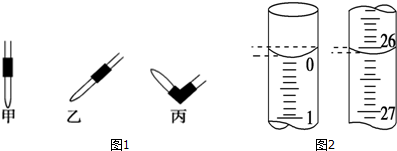
����ȥ��ʽ�ζ��������ݵķ���Ӧ������ͼ1��ʾ�����еı���Ȼ�����ἷѹ������ʹ���첿�ֳ�����Һ��
�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ2��ʾ������ʼ����Ϊ0.00 mL���յ����Ϊ26.10 mL������NaOH��Һ�����Ϊ26.10 mL��
��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���DE������ĸ��ţ���
A���ζ��յ����ʱ����
B����ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C����ƿˮϴ��δ����
D������NaOH�������Na2CO3����
E����ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ��
��1������1���ȼ�������ϡ���ᣬ�ټ���˫��ˮ����˫��ˮ����÷�Ӧ�����ӷ���ʽ��2Fe2++H2O2+2H+�T2Fe3++2H2O��
��2������ڵ�����ҺpH������ѡ�õ��Լ���BC��������ĸ��ţ�
A��A12O3 B��CuO C��CuCO3•Cu��OH��2
��3���й��������↑ʼ��������ȫ������pH���±���
| �������� | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Cu��OH��2 |
| ��ʼ������pH | 3.3 | 1.5 | 6.5 | 4.2 |
| ������ȫ��pH | 5.2 | 3.7 | 9.7 | 6.7 |
��4������ҺBͨ������Ũ������ȴ�ᾧ�����˵Ȳ����ɵõ�����ͭ���壮
��5������ͭҲ������ͭ�������ڸ��¡����������»����Ƶã��÷�Ӧ�Ļ�ѧ����ʽ��CuS+2O2$\frac{\underline{\;\;\;����\;\;\;}}{����}$CuSO4��ȡ384g CuS��һ�������º�������ȫ��Ӧ��������2CuS+3O2=2CuO+2SO2��4CuS+5O2=2Cu2O+4SO2������Ӧ�������ù�����Cu��O�����ʵ���֮��n��Cu����n��O��=4��a����ʱ���Ŀ��������ʵ���Ϊbmol����a=$\frac{2}{5}$b-8��������ռ������������֮һ��
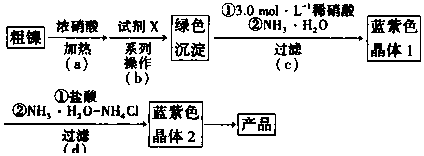
��֪���ٲ������������������������pH����ʼ������pH������Ũ��Ϊ0.1mol•L-1���㣩�����ʾ��
| Fe3+ | Cr3+ | Ni2+ | |
| ��ʼ����pH | 1.5 | 4.3 | 6.9 |
| ��ȫ����pH | 2.8 | 5.6 | 8.9 |
�ش��������⣺
��1��ʵ����Ҫ����3.0mol•L-1ϡ����250mL����Ҫ�IJ����������ձ�����Ͳ������������ͷ�ιܺ�250mL����ƿ��
��2�����裨a����Ni��Ũ���ᷴӦ�Ļ�ѧ����ʽΪNi+4��Ũ��HNO3$\frac{\underline{\;\;��\;\;}}{\;}$Ni��NO3��2+2NO2��+2H2O��
��3�����裨b�����ȼ����Լ�x������Һ��pHԼΪ6�����˺��ټ�������X����pH�Եõ���ɫ������
�ٵ���pHԼΪ6��ԭ����ʹCr3+��Fe3+��ȫ��������Ni2+δ��ʼ����
���Լ�X������C�����ţ���
A��H2SO4 B��Ni��OH��2 C��NaOH D��Fe2O3 E��NiO
��4�����裨c���ͣ�d���еķ�Ӧ����Ҫ�ڱ�ˮԡ�����½��У������ó��˿��Լ��ٰ�ˮ�Ļӷ������н����¶��Խ��Ͳ�����ܽ�ȶ�����
��5��NH3�����IJⶨ��[��֪��Ni��NH3��6Cl2+6HCl=NiCl2+6NH4Cl]
i���õ�����ƽ����mg��Ʒ����ƿ�У���25mLˮ�ܽ�����5mL��6mol•L-1���ᣬ�Լ�����ָʾ�����ζ����յ�����0.500 0mol•L-1 NaOH����ҺV1mL��
ii���հ����飺��������Ʒ�ظ�ʵ��i������NaOH����ҺV2 mL��
��NH3����������Ϊ$\frac{��V{\;}_{2}-V{\;}_{1}����10{\;}^{-3}��8.5}{m}$��100%��
�������������Ļ����ϣ����д�ʩ���ܽ�һ����߲ⶨȷ�ȵ���AD�����ţ���
A���ʵ���߳�����Ʒ������ B����H2SO4��Һ�������C���÷�̪������� D������ƽ�����飮