��Ŀ����
����ͭ�����ܽ����Һ������ɫ���μӰ�ˮֱ�����������յõ�����ɫ����Һ���ټ�����ˮ�Ҵ�����������ɫ���壬��ѧʽΪ��CuSO4?4NH3?H2O�����������ʵ������ش��������⣺
��1��д�������CuSO4?4NH3?H2O�е����壺 ����λ���ǣ� �����û�ѧʽ�����ֱ�ʾ����N��O��S����Ԫ�صĵ�һ�����ܴӴ�С��˳���� ��
��2����֪CuSO4?4NH3?H2O�Ľṹ��CuSO4?5H2O�������ƣ�CuSO4?5H2O����Ľṹ��ͼ��ʾ���뻭��CuSO4?4NH3?H2O�Ľṹʾ��ͼ�� ��
��
��3����CuSO4?4NH3?H2O�����У�����ԭ����sp3�ӻ���ԭ���У� ��дԪ�ط��ţ����侧��ṹ�д��ڵĻ�ѧ���У� ��
A�����»��� B�������� C�����ۼ� D����� E�����Ӽ� F����λ����
��1��д�������CuSO4?4NH3?H2O�е����壺
��2����֪CuSO4?4NH3?H2O�Ľṹ��CuSO4?5H2O�������ƣ�CuSO4?5H2O����Ľṹ��ͼ��ʾ���뻭��CuSO4?4NH3?H2O�Ľṹʾ��ͼ��

��3����CuSO4?4NH3?H2O�����У�����ԭ����sp3�ӻ���ԭ���У�
A�����»��� B�������� C�����ۼ� D����� E�����Ӽ� F����λ����
���㣺�����ijɼ����,Ԫ�ص����ܡ��縺�Եĺ��弰Ӧ��,��ѧ��,ԭ�ӹ���ӻ���ʽ���ӻ������ж�
ר�⣺��ѧ���뾧��ṹ
��������1������ͭ�����ܽ����Һ������ɫ����Һ���γ�����ˮ��ͭ�����ӣ��μӰ�ˮֱ�����������յõ�����ɫ����Һ����Һ����ˮ��ͭ�����ӱ�����İ���ͭ�����ӣ�������CuSO4?4NH3?H2O�е�����Ϊ�ĸ������ӣ�����N��O��S����Ԫ�������ڱ��е�λ�ã������ƶϳ����ǵ�һ�����ܵĴ�С��
��2�����ݽṹͼ��֪��ͭ���ӵ���λ��Ϊ4����CuSO4?5H2O�������ĸ��������ˮ���ӻ��ɰ����Ӽ��ɵ�CuSO4?4NH3?H2O�Ľṹʾ��ͼ���ݴ˴��⣻
��3�����ݷ���������ԭ�ӵļ۲���Ӷ����жϣ����ݾ���ṹ�������������������
��2�����ݽṹͼ��֪��ͭ���ӵ���λ��Ϊ4����CuSO4?5H2O�������ĸ��������ˮ���ӻ��ɰ����Ӽ��ɵ�CuSO4?4NH3?H2O�Ľṹʾ��ͼ���ݴ˴��⣻
��3�����ݷ���������ԭ�ӵļ۲���Ӷ����жϣ����ݾ���ṹ�������������������
���
�⣺��1������ͭ�����ܽ����Һ������ɫ����Һ���γ�����ˮ��ͭ�����ӣ��μӰ�ˮֱ�����������յõ�����ɫ����Һ����Һ����ˮ��ͭ�����ӱ�����İ���ͭ�����ӣ�������CuSO4?4NH3?H2O�е�����Ϊ�ĸ������ӣ�����N��O��S����Ԫ�������ڱ��е�λ�ã�O��NԪ�ض��ǵڶ����ڷǽ���Ԫ�أ�ͬһ����Ԫ��������ҵ�һ�����ܳ��������ƣ���NԪ��ԭ��2p�ܼ��ǰ����ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ�O��S��ͬһ���壬ͬһ�������϶��µ�һ�����ܳʼ�С���ƣ�����N��O��S����Ԫ�صĵ�һ�����ܴӴ�С��˳����N��O��S���ʴ�Ϊ��NH3��4��N��O��S��
��2�����ݽṹͼ��֪��ͭ���ӵ���λ��Ϊ4����CuSO4?5H2O�������ĸ��������ˮ���ӻ��ɰ����Ӽ��ɵ�CuSO4?4NH3?H2O�Ľṹʾ��ͼΪ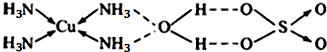 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��CuSO4��S��O�γ�4���Ҽ���û�й¶Ե��ӣ���sp3�ӻ���NH3��N��H�γ�3���Ҽ�����1�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����sp3�ӻ���H2O��O��H�γ�2���Ҽ�����2�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����sp3�ӻ���������ԭ����sp3�ӻ���ԭ����N��O��S��CuSO4?4NH3?H2O�У�Cu��NԪ�ؼ��γ���λ����H��N֮���γɹ��ۼ���Cu�����������֮���γ����Ӽ���
�ʴ�Ϊ��N��O��S��CEF��
��2�����ݽṹͼ��֪��ͭ���ӵ���λ��Ϊ4����CuSO4?5H2O�������ĸ��������ˮ���ӻ��ɰ����Ӽ��ɵ�CuSO4?4NH3?H2O�Ľṹʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��CuSO4��S��O�γ�4���Ҽ���û�й¶Ե��ӣ���sp3�ӻ���NH3��N��H�γ�3���Ҽ�����1�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����sp3�ӻ���H2O��O��H�γ�2���Ҽ�����2�Թ¶Ե��ӣ���۲���Ӷ���Ϊ4����sp3�ӻ���������ԭ����sp3�ӻ���ԭ����N��O��S��CuSO4?4NH3?H2O�У�Cu��NԪ�ؼ��γ���λ����H��N֮���γɹ��ۼ���Cu�����������֮���γ����Ӽ���
�ʴ�Ϊ��N��O��S��CEF��
���������⿼���������Ľṹ���ɼ��������һ�����ܵ�֪ʶ���ѶȲ�����ʱҪѧ��Ի���֪ʶ��������ã�

��ϰ��ϵ�д�
�����Ŀ
�������ĺ�������Ų�������ǣ�������
A�� |
B�� |
C�� |
D�� |
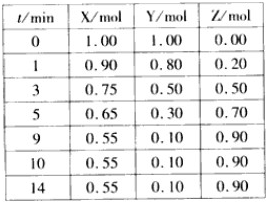
 ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ��
ij�¶�ʱ����2L�ܱ���������̬����X��Y��Ӧ������̬����Z�����ǵ����ʵ�����ʱ��ı仯�����ʾ��