��Ŀ����
13��ˮ��Ⱦ�����к��������ˮ��ʹ�ü�ֵ���ͻ�ɥʧ��ˮ����Ⱦ����Ҫ��Դ�й�ҵ��ˮ��������ˮ����1��������ˮ�е��ף���Ҫ��H3PO3��H3PO4��ʽ���ڣ�������������Ҫ��һ�ֹؼ�Ԫ�أ�����ɺ���������Ӫ�����ͺ�����ֳೱ����Ҫԭ��
��֪��������H3PO3��һ�ֶ�Ԫ��ǿ�ᣬ���н�ǿ��ԭ�ԣ������ڻ�ѧ�ƣ�
��H3PO3����Ԫ�صĻ��ϼ�Ϊ+3�ۣ�
��д��H3PO3������NaOH��Һ��Ӧ�����ӷ���ʽH3PO3+2OH-=HPO32-+2H2O��
��H3PO3���Խ���Һ�е�Ni2+��ԭ���Ӷ����ڻ�ѧ������д���÷�Ӧ�����ӷ���ʽH3PO3+Ni2++H2O=H3PO4+Ni+2H+��
��2����ҵ��ˮ�а��������ؽ������ӡ����������CN-�ȣ�
��֪��HCN�ĵ��볣��K=5��10-10��
��NaCN��Һ�ʼ��ԣ���ԭ����CN-+H2O?HCN+OH-�������ӷ���ʽ��ʾ����
������2mol/L HCN��Һ��1mol/L NaOH��Һ�������ϣ�������Һ��pHԼΪ9.3����֪lg5=0.7 ����
��NaCN�о綾����NaCN�ķ�ˮ�������ŷţ�ȷ����ˮ��NaCN�ĺ����Ƿ�ﵽ�ŷű�������AgNO3��Һ�ζ����ζ�ʱAgNO3��ҺӦʢ������ʽ�ζ��ܣ����������ƣ�����֪��2CN-+Ag+�TAg��CN��2-��I-+Ag+�TAgI��������AgI�ʻ�ɫ��CN-������Ag+��Ӧ����ʵ����ʹ��KI��ָʾ�����ﵽ�ζ��յ�ʱ���������л�ɫ�������ɣ�
���� ��1���ٻ�������Ԫ�ػ��ϼ۴�����Ϊ0��
��������H3PO3��һ�ֶ�Ԫ��ǿ�ᣬ������NaOH��Һ��Ӧ����NaHPO3��ˮ��
��H3PO3���Խ���Һ�е�Ni2+��ԭ�Ӷ����ڻ�ѧ������˵�������ӱ���ԭΪ�������ʣ������ᱻ����Ϊ���
��2����HCN�ĵ��볣��K=5��10-10��HCNΪ���ᣬNaCN��Һ��ǿ����������Һ��CN-����ˮ��ʼ��ԣ�
������2mol/L HCN��Һ��1mol/L NaOH��Һ�������ϵõ���Ũ�ȵ�NaCN��HCN�Ļ����Һ�������Һ��HCN��Ũ��Ϊ0.5mol/L��NaCNŨ��Ϊ0.5mol/L�����HCN�ĵ���ƽ�ⳣ�����㣻
�۱�AgNO3��Һ�������ԣ�Ӧѡ����ʽ�ζ���ʢ�ţ�Ag+��CN-��Ӧ����[Ag��CN��2]-����CN-��Ӧ����ʱ��Ag+��I-����AgI��ɫ������˵����Ӧ����ζ��յ㣻
��� �⣺��1����H3PO3����Ԫ�ػ��ϼ�+1�ۣ���Ԫ�ػ��ϼ�-2�ۣ�Ԫ�ػ��ϼ۵�����Ϊ0�õ���Ԫ�صĻ��ϼ�Ϊ+3�ۣ�
�ʴ�Ϊ��+3�ۣ�
��������H3PO3��һ�ֶ�Ԫ��ǿ�ᣬ������NaOH��Һ��Ӧ����NaHPO3��ˮ����Ӧ�����ӷ���ʽΪ��H3PO3+2OH-=HPO32-+2H2O��
�ʴ�Ϊ��H3PO3+2OH-=HPO32-+2H2O��
��������H3PO3��һ�ֶ�Ԫ��ǿ�ᣬ���н�ǿ��ԭ�ԣ�H3PO3���Խ���Һ�е�Ni2+��ԭ�Ӷ����ڻ�ѧ������˵�������ӱ���ԭΪ�������ʣ������ᱻ����Ϊ���ᣬ��Ӧ�����ӷ���ʽΪ��H3PO3+Ni2++H2O=H3PO4+Ni+2H+��
�ʴ�Ϊ��H3PO3+Ni2++H2O=H3PO4+Ni+2H+��
��2����HCN�ĵ��볣��K=5��10-10��HCNΪ���ᣬNaCN��Һ��ǿ����������Һ��CN-����ˮ��ʼ��ԣ����ӷ���ʽΪ��CN-+H2O?HCN+OH-��
�ʴ�Ϊ��CN-+H2O?HCN+OH-��
������2mol/L HCN��Һ��1mol/L NaOH��Һ�������ϵõ���Ũ�ȵ�NaCN��HCN�Ļ����Һ�������Һ��HCN��Ũ��Ϊ0.5mol/L��NaCNŨ��Ϊ0.5mol/L��Ka=$\frac{c��C{N}^{-}��c��{H}^{+}��}{c��HCN��}$=5��10-10��HCN?H++CN-��
c��H+��=$\frac{5��1{0}^{-10}��0.5}{0.5}$=5��10-10mol/L
PH=10-lg5=9.3��
�ʴ�Ϊ��9.3��
�۱�AgNO3��Һ�������ԣ�Ӧѡ����ʽ�ζ���ʢ�ţ�Ag+��CN-��Ӧ����[Ag��CN��2]-����CN-��Ӧ����ʱ��Ag+��I-����AgI��ɫ������˵����Ӧ����ζ��յ㣻
�ʴ�Ϊ����ʽ�ζ��ܣ� �л�ɫ�������ɣ�
���� ���⿼�����ӷ���ʽ��д������ˮ�⡢�����Ʊ�ʵ�顢���ʺ����ⶨ�ȣ���Ŀ�ۺ��Խ�ǿ���ؼ��Ƕ�ԭ�������⼰ʵ������Ĺ淶�ԣ���������Ԫ�ػ�����֪ʶ��ʵ���Ʊ�����ԭ����Ŀ�Ѷ��еȣ�

 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�| A�� |  ��ͼ��ʾSO2������Ӧ�ֱ����С�����������·�Ӧ�����е������仯 | |
| B�� |  ��ͼ��ʾ0.1molMgCl2•6H2O�ڿ����г�ּ���ʱ����������ʱ��ı仯 | |
| C�� |  ��ͼ��ʾ�ֱ�ϡ��1mLpH=2������ʹ���ʱ��ҺpH�ı仯��ͼ��b��100mL | |
| D�� |  ��ͼ��ʾƽ��2NO2��g��?N2O4��g����t1ʱѸ�ٽ������С��c��N2O4���ı仯 |
| A�� | ����������������� | B�� | ����һ������Ӻ�һ�������ӹ��� | ||
| C�� | ����������Ԫ�غ�������Ԫ�ع��� | D�� | ������Ԫ�غ���Ԫ����� |
| A�� | ����£�1 molˮ����ռ�е����Ϊ 22.4L | |
| B�� | 17g OH-�����������ȵ��������� NA | |
| C�� | ���ʵ���Ũ��Ϊ 0.5mol/L�� MgCl2��Һ�У����� Cl-����ĿΪ NA | |
| D�� | 4g������������ԭ�ӵ���ĿΪ NA |
| A�� | ���ȵ�ͭ˿���Ժ��Ҵ������û���Ӧ�������� | |
| B�� | ������ϩ����ʹ��ˮ��ɫ����ԭ����ͬ | |
| C�� | ���ۺ�����������һ�������¶��ܷ���ˮ�ⷴӦ | |
| D�� | �Ҵ�������������������ñ���Na2CO3��Һ���� |
| A�� | pH��ֱֽ��պȡNaOH��Һ�ⶨ��pH | |
| B�� | ��Ż�ѧƷ�IJֿ�ʧ�������ˮ��� | |
| C�� | ����ʱ������һ��ʱ�����Ͷ���ʯ | |
| D�� | �и�ʣ�µİ��Ż�ԭ�Լ�ƿˮ�Ᵽ�� |
| A�� | Ũ���������ˮ�ԣ������ʹ����̿�� | |
| B�� | Ũ�����ڳ����¿�Ѹ����ͭƬ��Ӧ�ų������������� | |
| C�� | Ũ���������ˮ�ԣ���˿�������������ܸ������������⡢���������� | |
| D�� | ��ΪŨ�����ڳ����²�������������Ӧ����˳����¿������ƻ�������������Ũ���� |
 �й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����A�ǽ������ʣ�D�Ƿǽ������ʣ�B��F��������B��ɫ��ζ��G���������ɫҺ�壮��ش��������⣺
�й����ʵ�ת����ϵ��ͼ��ʾ���������ʺ���������ȥ����A�ǽ������ʣ�D�Ƿǽ������ʣ�B��F��������B��ɫ��ζ��G���������ɫҺ�壮��ش��������⣺ ��
��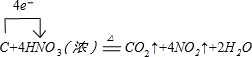 ��
��