��Ŀ����
��98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3������100mL 1.0mol/Lϡ���ᣬ��ʵ�������У�
A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι� G��50mL�ձ� H��100mL����ƿ
��1������ȡŨ��������Ϊ mL��
��2��ʵ��ʱѡ�õ������У�����ţ�
��3�����ƹ����У����������ʹ���ƽ��ƫ�ߵ��ǣ�����ţ�
�ٶ���ʱ���ӿ̶��߹۲�Һ��
������ƿʹ��ʱδ����
�۶��ݺ�ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
��4��������ƿʹ�÷����У����в�������ȷ���ǣ�����ţ�
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô���Һ��ϴ
C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ⣬����ע������ƿ��
D����ȷ��ȡ��18.4mol?L-1�����ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��5����ʵ������г����������Ӧ��δ�����
�ټ�����ˮ����̶���Լ1--2����ʱ��Ӧ
�ڼ�����ˮʱ���������˿̶��ߣ�Ӧ ��
A��100mL��Ͳ B��������ƽ C�������� D��50mL����ƿ
E��10mL��Ͳ F����ͷ�ι� G��50mL�ձ� H��100mL����ƿ
��1������ȡŨ��������Ϊ
��2��ʵ��ʱѡ�õ������У�����ţ�
��3�����ƹ����У����������ʹ���ƽ��ƫ�ߵ��ǣ�����ţ�
�ٶ���ʱ���ӿ̶��߹۲�Һ��
������ƿʹ��ʱδ����
�۶��ݺ�ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶���
��4��������ƿʹ�÷����У����в�������ȷ���ǣ�����ţ�
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô���Һ��ϴ
C�����������ƹ��������ƽ���̵���ֽ�ϣ�ȷ�����������ձ����ܽ⣬����ע������ƿ��
D����ȷ��ȡ��18.4mol?L-1�����ᣬע����ʢ��30mLˮ��100mL������ƿ�У���ˮ���̶���
E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ��
��5����ʵ������г����������Ӧ��δ�����
�ټ�����ˮ����̶���Լ1--2����ʱ��Ӧ
�ڼ�����ˮʱ���������˿̶��ߣ�Ӧ
���㣺����һ�����ʵ���Ũ�ȵ���Һ
ר�⣺ʵ����
��������1������C=
����Ũ��������ʵ���Ũ�ȣ�Ȼ�����ݼ�����ҪŨ����������
��2����Ũ��Һ����ϡ��Һһ���õ��������У���Ͳ�����������ձ�������ƿ����ͷ�ιܣ�����Ũ����������������Һ�����ѡ����ʵ���Ͳ������ƿ��
��3������C=
����������������ʹnƫС����Vƫ��IJ�������ʹ������ҺŨ��ƫС����֮��ƫ��
��4����������ƿ�Ĺ��켰��ȷ��ʹ�÷��������жϣ�
��5��������ƿƿ��ϸ��������ʱӦע�ⲻ�ܼ�������ˮ���ࣻ
�ڼ�����ˮʱ���������˿̶��ߣ�ʵ��ʧ�ܣ������²�����
| 1000�� |
| M |
��2����Ũ��Һ����ϡ��Һһ���õ��������У���Ͳ�����������ձ�������ƿ����ͷ�ιܣ�����Ũ����������������Һ�����ѡ����ʵ���Ͳ������ƿ��
��3������C=
| n |
| V |
��4����������ƿ�Ĺ��켰��ȷ��ʹ�÷��������жϣ�
��5��������ƿƿ��ϸ��������ʱӦע�ⲻ�ܼ�������ˮ���ࣻ
�ڼ�����ˮʱ���������˿̶��ߣ�ʵ��ʧ�ܣ������²�����
���
��1��98%��Ũ���ᣨ���ܶ�Ϊ1.84g/cm3�������ʵ���Ũ��C=
=
18.4mol/L��
ϡ��ǰ�����ʵ����ʵ������ֲ��䣬����ҪŨ��������ΪV����18.4mol/L��V=100mL��1.0mol/L�����V=5.4ml��
�ʴ�Ϊ��5.4��
��2����Ũ��Һ����ϡ��Һһ���õ��������У���Ͳ�����������ձ�������ƿ����ͷ�ιܣ�Ҫ����100mL��ҺӦѡ��100ml������ƿ��������ҪŨ��������Ϊ5.4ml����֪Ӧѡ��10ml��Ͳ��
�ʴ�Ϊ��C E F G H��
��3������C=
����������������ʹnƫС����Vƫ��IJ�������ʹ������ҺŨ��ƫС����֮��ƫ��
�ٶ���ʱ���ӿ̶��߹۲�Һ�棬���¼��������ˮ���٣���Һ�����VƫС����Һ��Ũ��ƫ�ʢ�ѡ��
������ƿʹ��ʱδ�������Һ����������ʵ������������Ӱ�죬��ҺŨ��ȷ���ʢڲ�ѡ��
�۶��ݺ�ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߣ���������ˮ���࣬��Һ�����Vƫ����Һ��Ũ��ƫС���ʢ۲�ѡ��
��ѡ���٣�
��4��A��ʹ��D��ƿ��������������Ӧ������Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ�������ô���Һ��ϴ�����¼�������ʹ��࣬Ũ��ƫ��B����
C���������ƹ�����и�ʴ�ԣ�Ӧ�÷����ձ��г�����ȷ�����������ձ����ܽ⣬��ȴ������������ƿ�У���C����
D������ƿΪ������������������ϡ����Һ�����ܽ���壬��D����
E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ�ȣ���E��ȷ��
��ѡ��BCD��
��5���ټ�����ˮ����̶���Լ1--2����ʱ��Ӧ�ã����ý�ͷ�ιܵ�����Һ����͵���̶������У�
�ڼ�����ˮʱ���������˿̶��ߣ������������ƣ�
�ʴ�Ϊ�����ý�ͷ�ιܵ�����Һ����͵���̶������У������������ƣ�
| 1000�Ѧ� |
| M |
| 1000��1.84g/cm3��98% |
| 98g/mol |
ϡ��ǰ�����ʵ����ʵ������ֲ��䣬����ҪŨ��������ΪV����18.4mol/L��V=100mL��1.0mol/L�����V=5.4ml��
�ʴ�Ϊ��5.4��
��2����Ũ��Һ����ϡ��Һһ���õ��������У���Ͳ�����������ձ�������ƿ����ͷ�ιܣ�Ҫ����100mL��ҺӦѡ��100ml������ƿ��������ҪŨ��������Ϊ5.4ml����֪Ӧѡ��10ml��Ͳ��
�ʴ�Ϊ��C E F G H��
��3������C=
| n |
| V |
�ٶ���ʱ���ӿ̶��߹۲�Һ�棬���¼��������ˮ���٣���Һ�����VƫС����Һ��Ũ��ƫ�ʢ�ѡ��
������ƿʹ��ʱδ�������Һ����������ʵ������������Ӱ�죬��ҺŨ��ȷ���ʢڲ�ѡ��
�۶��ݺ�ҡ�ȡ����ã�����Һ����ڿ̶��ߣ��ټ�����ˮ�����̶��ߣ���������ˮ���࣬��Һ�����Vƫ����Һ��Ũ��ƫС���ʢ۲�ѡ��
��ѡ���٣�
��4��A��ʹ��D��ƿ��������������Ӧ������Ƿ�©ˮ����A��ȷ��
B������ƿ������ˮϴ�������ô���Һ��ϴ�����¼�������ʹ��࣬Ũ��ƫ��B����
C���������ƹ�����и�ʴ�ԣ�Ӧ�÷����ձ��г�����ȷ�����������ձ����ܽ⣬��ȴ������������ƿ�У���C����
D������ƿΪ������������������ϡ����Һ�����ܽ���壬��D����
E�����ݺ�����ƿ������ʳָ��סƿ��������һֻ�ֵ���ָ��סƿ�ף�������ƿ��תҡ�ȣ���E��ȷ��
��ѡ��BCD��
��5���ټ�����ˮ����̶���Լ1--2����ʱ��Ӧ�ã����ý�ͷ�ιܵ�����Һ����͵���̶������У�
�ڼ�����ˮʱ���������˿̶��ߣ������������ƣ�
�ʴ�Ϊ�����ý�ͷ�ιܵ�����Һ����͵���̶������У������������ƣ�
���������⿼����һ�����ʵ���Ũ����Һ�����ã���ȷ���õ�ԭ�����������õIJ����ǽ���ؼ���ע����Ͳ������ƿ��ѡ��ԭ����ѭ�����������

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ
�������ӷ���ʽ��д��ȷ���ǣ�������
| A��̼��������Һ�м��������ʯ��ˮ��Ca2++2HCO3-+2OH-�TCaCO3��+CO32-+2H2O |
| B���Ȼ�����Һ�м��������ˮ��Al3++4NH3?H2O�TAlO2-+4NH4++2H2O |
C����������Һ��ͨ������������̼�� |
| D������ˮ��Ӧ��2Na+2H2O�T2Na++2OH-+H2�� |
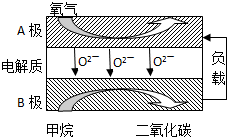 ��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�
��������Ȼ������Ҫ�ɷ֣�������������Ӧ�÷dz��㷺��һ�ֻ�ѧ���ʣ�