��Ŀ����
8��ijһ����Һ�����ܺ���NH4+��K+��Ba2+��Al3+��Fe3+��I-��NO3-��CO32-��SO42-��AlO2-��ȡ����Һ��������ʵ�飺����pH��ֽ���飬��Һ��ǿ���ԣ�
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ�
b����Һ����������
c��������ȫ�ܽ⣻
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��ȡ�����۵õ��ļ�����Һ����Na2CO3��Һ���а�ɫ�������ɣ�
��������ʵ�����ش��������⣮
��1���ɢٿ����ų�CO32-��AlO2-���ӵĴ��ڣ�
��2���ɢڿ���֤��I-���ӵĴ��ڣ�ͬʱ�ų�Fe3+��NO3-���Ӵ��ڣ�
��3���ɢۿ�֤��Al3+��NH4+���Ӵ��ڣ�
��4���ɢܿ����ų�SO42-���Ӵ��ڣ�
���� ����pH��ֽ��⣬��Һ��ǿ���ԣ�˵����Һ�д���H+���������ӹ����֪һ��������CO32-��AlO2-��
��ȡ��Һ��������������CCl4������������ˮ����CCl4����Ϻ�ɫ��˵����Һ��һ������I-���������ӹ�������жϿ�֪��һ��������Fe3+��NO3-��
����ȡ��Һ��������μ���NaOH��Һ��
a����Һ�����Ա�Ϊ���ԣ��к��
b����Һ����������������������ֻ�������ӳ�����
c��������ȫ�ܽ⣬֤�����ɵij���������������ԭ��Һ��һ������Al3+��
d����������Һ��������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������֤�����ɵ������ǰ�����ԭ��Һ��һ������笠����ӣ�
��ȡ�����۵õ��ļ�����Һ�������ӷ�Ӧ����ƫ��������ӣ�����Na2CO3��Һ���а�ɫ�������ɣ��������ӿ�ֻ֪�б���������̼�ᱵ������˵��ԭ��Һ�в�������������ӣ�
�����ƶ����ӵĴ�������ش����⣮
��� �⣺����pH��ֽ���飬��Һ��ǿ���ԣ���Һ�д��ڴ���H+������̼������Ӻ�ƫ��������Ӷ������������Ӻ������ӷ�Ӧ������CO32-��AlO2- ���ܴ������ڣ�
��ȡ��Һ��������������CCl4���������Ƶ���ˮ�����Ȼ�̼�����ɫ��˵����Һ�к���I-������ Fe3+��NO3-��������Һ�У�������I-ΪI2��������Һ�в�����Fe3+��NO3-��
�������������ɵij������ܽ⣬˵����Һ�д���Al3+�����ݼ�����Һ���ɵ�������ʹʪ��ĺ�ɫʯ����ֽ����֤�������ǰ�����˵��ԭ��Һ�к���NH4+��
��ȡ�����۵õ��ļ�����Һ������̼������Һ�а�ɫ�������ɣ�˵����Һ�д��ڱ����ӣ���һ����������������ӣ�
��1���ɢٿ����ų�CO32-��AlO2-�Ĵ��ڣ��ʴ�Ϊ��CO32-��AlO2-��
��2���ɢڿ���֤��I-һ���Ĵ��ڣ�CCl4����ֵⵥ�ʵ���ɫ֤����I-��Fe3+��NO3-�ڸû�������I-���ܹ��棬�������ӹ���ͬʱ�ų�Fe3+��NO3-���ӵĴ��ڣ�
�ʴ�Ϊ��I-��Fe3+��NO3-��
��3����֤����Һ��һ������Al3+��NH4+���ʴ�Ϊ��Al3+��NH4+��
��4����ʵ������жϣ�ȡ�����۵õ��ļ�����Һ�������ӷ�Ӧ����ƫ��������ӣ�����Na2CO3��Һ���а�ɫ�������ɣ��������ӿ�ֻ֪�б���������̼�ᱵ������˵��ԭ��Һ�в�������������ӣ�
�ʴ�Ϊ��SO42-��
���� ���⿼�������Ӽ����ʵ�鷽���ͷ�Ӧ������жϣ��������ʺ����ӷ�Ӧ���е����ӹ�������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | c��H+��=c��CH3COO-�� | |
| B�� | ��Һ�У�c��H+����c��OH-�� | |
| C�� | CH3COOH���ӵ�Ũ�Ȳ��ٱ仯 | |
| D�� | ��Һ��ͬʱ����H+��CH3COO-��OH-��CH3COOH��H2O |
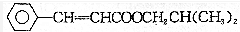 �������й�˵���д�����ǣ�������
�������й�˵���д�����ǣ�������| A�� | �ڼ���Һ���ױ��� | |
| B�� | ��ʹ����KMnO4��Һ��ɫ | |
| C�� | ����ʽΪC13H16O2 | |
| D�� | ��HClǡ����ȫ�ӳ�ʱ�ɵõ�һ�ִ����� |
��֪��CH3OH��l��+O2��g��=CO��g��+2H2O��g����H=-443.64kJ•mol-1
2CO��g��+O2��g��=2CO2��g����H2=-566.0kJ•mol-1
����˵�����Ȼ�ѧ����ʽ��ȷ���ǣ�������
| A�� | CO��ȼ����Ϊ566.0 kJ•mol-1 | |
| B�� | 2 mol CO��1 mol O2����������2 mol CO2���������� | |
| C�� | ��ȫȼ��20g�״������ɶ�����̼��Һ̬ˮʱ�ų�������Ϊ908.3 kJ | |
| D�� | 2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g��a=-1453.28 kJ•mol-1 |
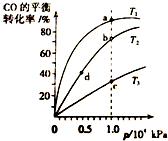 ��CO�ϳɼ״��ķ�ӦΪ��CO��g��+2H2��g���TCH3OH ��g����H��0��������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��CO�ϳɼ״��ķ�ӦΪ��CO��g��+2H2��g���TCH3OH ��g����H��0��������ͬ�����ʵ���Ͷ�ϣ����CO�ڲ�ͬ�¶��µ�ƽ��ת������ѹǿ�Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �¶ȣ�T1��T2��T3 | B�� | ����Ӧ���ʣ�v��a����v��c������ v��b����v��d�� | ||
| C�� | ƽ�ⳣ����K��a����K��b�� K��b��=K��d�� | D�� | ƽ��Ħ��������M��a����M��c������ M��b����M��d�� |
| A�� | Mg | B�� | Al | C�� | Si | D�� | S |