��Ŀ����
19��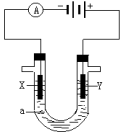 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ�������ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ�������ش��������⣺��1����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ������
�����뼸�η�̪��Һ���� X�������۲쵽�������ǣ������ݣ���Һ���
��2��Y�缫�ϵĵ缫��Ӧʽ��2Cl--2e-=Cl2��������õ缫��Ӧ����ķ����ǣ���ʪ��ĵ��۵⻯����ֽ�ӽ������ڣ������ֽ����ɫ��˵����������
��3������õ�ⷽ��������ͭ����ͭ�к�������п�����������Һaѡ��CuSO4��Һ����Y �缫�IJ����Ǵ�ͭ���缫��Ӧʽ��Zn-2e�TZn2+��Cu-2e-�TCu2+��
���� ��1����ⱥ��ʳ��ˮʱ���ɵ�Դ��֪��XΪ������YΪ��������������������ʧ���ӣ��������������ӵõ��ӣ��Դ˽����⣻
��2��Y�缫�������ӷŵ���������������������ʪ��ĵ��۵⻯����ֽ���飻
��3����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ��������ͭ���ӷŵ磮
��� �⣺��1���͵�Դ�ĸ��������ĵ缫X�����������õ缫�������ӷ����õ��ӵĻ�ԭ��Ӧ����2H++2e-=H2����������Χ�������������������ӣ����Եη�̪��Һ��죬���������ݲ�������Һ��죬�ʴ�Ϊ�������ݣ���Һ��죻
��2����X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��Y�缫�������ӷŵ������������缫��ӦʽΪ2Cl--2e-=Cl2�����������������ԣ����������������ɵⵥ�ʣ�����������Һ����ɫ����������������ʪ��ĵ��۵⻯����ֽ���飬
�ʴ�Ϊ��2Cl--2e-=Cl2������ʪ��ĵ��۵⻯����ֽ�ӽ������ڣ������ֽ����ɫ��˵������������
��3����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ�������缫��Ӧʽ��Zn-2e�TZn2+��Cu-2e-�TCu2+��������ͭ���ӷŵ磬�缫��ӦʽΪCu2++2e-=Cu��
�ʴ�Ϊ����ͭ��Zn-2e�TZn2+��Cu-2e-�TCu2+��
���� ���⿼��ԭ���ԭ�����漰�缫��Ӧʽ����д�������ļ����֪ʶ�㣬֪�����ӷŵ�˳�缫��Ӧʽ����д�����������ļ��鷽����������Ŀ�ѶȲ���

 ��У����ϵ�д�
��У����ϵ�д�| A�� | ��Ӧֹͣ�� | |
| B�� | NO������Ӧ������O2���淴Ӧ������� | |
| C�� | c��NO����c��O2��=2��1 | |
| D�� | �������ɫ���ٱ仯 |
| A�� | ����ʹ��̪��Һ������ɫ��Һ�У�Na+��CO32-��K+��ClO- | |
| B�� | �����������Ӧ�ų���������Һ�У�K+��NO3-��Cl-��NH4+ | |
| C�� | ������ˮ�������c��H+ ��•c��OH- ��=10-20����Һ�У�Na+��Cl-��S2-��SO32- | |
| D�� | ��ɫ����Һ��K+��HCO3-��NO3-��SO42-��Fe3+ |
I����С��̽���ھƾ���Ƽ���������FeSO4�ֽ��������ʵ��װ����ͼ��ʾ��
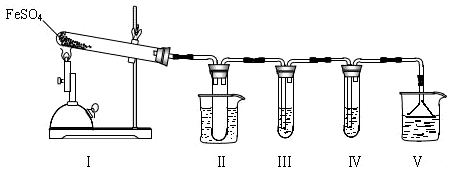
��1��װ�â���Թ��в�װ�κ��Լ����������Ƿ�ֹ��Һ������װ�â��У���ȫƿ�����Թܽ�����50���ˮԡ�У�Ŀ���Ƿ�ֹSO3Һ�������̣�
��2��װ�â��װ�â���������̽����ʵ���������ɷ֣������ʵ����ƣ���д�����Լ���Ԥ����������ۣ�
��ѡ�Լ���3mol•L-1 H2SO4��Һ��6mol•L-1 NaOH��Һ��0.5mol•L-1 BaCl2��Һ��0.5mol•L-1 Ba��NO3��2��Һ��0.01mol•L-1����KMnO4��Һ��0.01mol•L-1��ˮ��
| �����Լ� | Ԥ������ͽ��� |
| װ�â���Թ��м���0.5 mol•L -1 BaCl2�� | ����������ɫ������֤����������к���SO3�� |
| װ�â����Թ��м���0.01 mol•L-1 ���� KMnO4 ��Һ����0.0l mol•L-1 ��ˮ���� | ����Һ��ɫ�����ɫ����ȥ��֤����������к���SO2������Һ��ɫ�����ɫ�������Ա仯��֤����������в���SO2 |

��������̻ش�
��1���������б����õ���������A
A.25mL��ʽ�ζ��� B.50mL ��Ͳ C.25mL ��Ͳ D.25mL��ʽ�ζ���
��2��ϵ�в�����������Ϊ���ˡ�ϴ�ӡ����գ�
��3�������ղ�ú���ɫ��������Ϊ3.2g������Ʒ����Ԫ�ص���������Ϊ70%��
| A�� | ��˾ƥ���dz��õĿ���ҩ | |
| B�� | �̬���ʳ�������������ľ�ҵȻ��ʩ�� | |
| C�� | ��ʯ����Ҫ�ɷ������Ի����� | |
| D�� | �Ӻ�ˮ�п���ȡ�� |

