��Ŀ����
3��������ʵ������ó��Ľ�����ȷ���ǣ�������| ��� | ʵ���������� | ʵ����� |
| A | ��ij���Һ��ͬʱ�μӼ���KSCN��Һ��������������ˮ����Һ���Ѫ��ɫ | ����Һ��һ������Fe3+ |
| B | ������ʯ��ˮ������ܻ���Na2CO3�� NaHCO3��Һ�г��ְ�ɫ���� | ��Һ��һ������Na2CO3 |
| C | �����£���pH�Ʋⶨij��ҺNaHSO3��Һ�� pHԼΪ5.20 | ����Һ��HSO3-�ĵ���̶ȴ�������ˮ��̶� |
| D | �����£���ij��Һ�еμ�ϡNaOH��Һ��ʪ��ĺ�ʯ����ֽ�����Թܿڣ���ֽ������ | ����Һ��һ��������NH4+ |
| A�� | A | B�� | B | C�� | C | D�� | D |
���� A����������ǿ�����ԣ������������������������ӣ������Ӻ�KSCN��Һ��Ӧ��ʹ��Һ��Ѫ��ɫ��
B���������ƺ�̼���ơ�̼�����ƶ���Ӧ����̼��ư�ɫ������
C����������������ܷ��������ˮ�⣬����������������Һ�����ȷ����ˮ��͵���̶���Դ�С��
D��笠����Ӻ�ϡ��NaOH��Ӧ����һˮ�ϰ���������û�а������ɣ�
��� �⣺A����������ǿ�����ԣ������������������������ӣ������Ӻ�KSCN��Һ��Ӧ��ʹ��Һ��Ѫ��ɫ��Ӧ���ȼ�KCN���������ӣ�Ȼ�����ˮ�����Ƿ����������ӣ���A����
B���������ƺ�̼���ơ�̼�����ƶ���Ӧ����̼��ư�ɫ������Ӧ�����Ȼ��Ƽ���̼���ơ�̼�����ƣ���B����
C����������������ܷ��������ˮ�⣬����������������Һ�����ȷ����ˮ��͵���̶���Դ�С�������£���pH�Ʋⶨij��ҺNaHSO3��Һ�� pHԼΪ5.20����Һ�����ԣ�˵������Һ��HSO3-�ĵ���̶ȴ�������ˮ��̶ȣ���C��ȷ��
D��笠����Ӻ�ϡ��NaOH��Ӧ����һˮ�ϰ���������û�а������ɣ�������Ũ��NaOH��Һ����笠����ӣ���D����
��ѡC��
���� ���⿼�黯ѧʵ�鷽�����ۣ�Ϊ��Ƶ���㣬�漰���Ӽ��顢�����ˮ��̶���Դ�С�Ƚϡ����ʼ����֪ʶ�㣬��ȷʵ��ԭ�����������ʲ������ǽⱾ��ؼ����״�ѡ����AB��

 ����Ӣ��ϵ�д�
����Ӣ��ϵ�д�| A�� | CH2=CH2��CH2=CH-CH=CH2 | B�� | C4H8��C6H12 | ||
| C�� |  �ͣ�CH3��2CHCH��CH3��2 �ͣ�CH3��2CHCH��CH3��2 | D�� | C2H6��C4H10 |
| A�� | �ǽ����ԣ�Cl��I | B�� | ���ԣ�KOH��NaOH | ||
| C�� | ���ԣ�H2SO3��H2SO4 | D�� | ���H+������CO32-��Cl- |
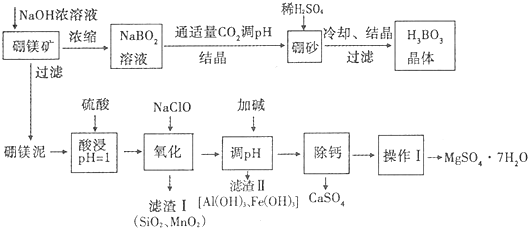
ע��
����þ�����Ҫ�ɷ���MgO��ռ40%��������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ�
���������ƽ�ⳣ��K=5.8��10-10
��
| ������ | Mg��OH��2 | Fe��OH��2 | Fe��OH��3 | Al��OH��3 |
| Ksp����ֵ | 10-11 | 10-16 | 10-38 | 10-33 |
| �¶ȣ��棩 | 40 | 50 | 60 | 70 |
| MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
| CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
��2����������ӿɱ�ʾΪB��OH��4-��д���������ķ���ʽH3BO3+H2O?B��OH��4-+H+
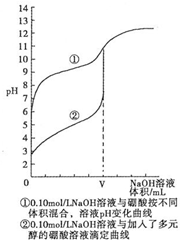
��3��N�ζ����ⶨ���ᾧ��Ĵ��ȣ��о������������0.1mol•L-1NaOH��Һֱ�ӵζ�������Һ���ζ����������ߢ���ʾ����������Һ�м����Ԫ�����ٵζ������ߢ���ʾ����������ܷ���ǿ��ֱ�ӵζ�������Һ������ ����ܡ����ܡ����������ǣ�û�����Ե�pHͻ���������ж��յ㣬����ɽϴ�����
��4������þ����������Һ�У������NaClO����Mn2+��Ӧ����MnO2�������÷�Ӧ�����ӷ���ʽ��ClO-+Mn2++H2O=MnO2��+Cl-+2H+��
��5���Ӽ������pHΪ4.7ʱ���������ӱ����ȫ������������Ũ��С�ڻ����1��l0-5mol•L-1ʱ��������Ϊ�����ӳ�����ȫ��
��6�������ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵�����ƵIJ�����������Ũ���ᾧ�����ȹ��ˣ�
| ��ѧʽ | CH3COOH | HClO | H2CO3 | H2C2O4 |
Ka | Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.1��10-7�� Ka2=5.6��10-11 | Ka1=5.9��10-2�� Ka2=6.4��10-5 |
| A�� | ��H2C2O4 ������ʵ����� KOH��Ӧ��������Һ�����ԣ������Һ�и�����Ũ���ɴ�С��˳��Ϊ��c��K+����c�� HC2O4-����c�� H+����c�� C2O42-����c�� OH-�� | |
| B�� | ̼������Һ�еμ�������ˮ�����ӷ���ʽΪ��CO32-+Cl2+H2O=Cl-+HClO+HCO3- | |
| C�� | �����£�0.1mol/LCH3COOH ��Һ��ˮϡ�����У�����ʽc�� H+��/c�� CH3COOH �������ݱ�� | |
| D�� | pH��ͬ�� NaClO �� CH3COOK ��Һ������Һ�����ʵ���Ũ�ȵĴ�С��ϵ�ǣ�c�� CH3COOK ����c�� NaClO �� |
| A�� | �����п����й������ | B�� | ������Һ�д���Fe2+��Fe3+��Cu2+ | ||
| C�� | ԭ������n��Fe2O3����n��Cu�� | D�� | �ټ���ͭ����Һ��ɫ����ȥ |
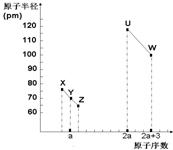 X��Y��Z��U��W���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ�Ӱ뾶��ԭ����������ͼ��ϵ��������XZ��ˮú������Ҫ�ɷ�֮һ������˵������ȷ���ǣ�������
X��Y��Z��U��W���ֶ����ڷǽ���Ԫ�أ����ǵ�ԭ�Ӱ뾶��ԭ����������ͼ��ϵ��������XZ��ˮú������Ҫ�ɷ�֮һ������˵������ȷ���ǣ�������| A�� | U��X��W ����Ԫ������������ˮ��������������ǿ | |
| B�� | ��Y��Z��������Ԫ���γɵĻ�������ֻ�й��ۼ� | |
| C�� | XZ2��YZ2��X60�Ļ�ѧ�����ͺ;������Ͷ���ͬ | |
| D�� | TԪ����Uͬ����������һ���ڣ����γɻ�����TW4��TZ2��T3Y4 |
 CH4��CO2���������ֵ���ߵĻ�ѧ��Ʒ����֪��
CH4��CO2���������ֵ���ߵĻ�ѧ��Ʒ����֪��