��Ŀ����
4��S2Cl2��һ����Ҫ�Ļ�����Ʒ������ʱ��һ���ж����ж���Ľ��ɫҺ�壬�۵�-76�棬�е�138�棬����ˮ��Ӧ����һ�������ɵ�SC12��SC12��ӣ�Һ�ɫҺ�壬�ӷ����۵�-122�棬�е�59�森SCl2��S2Cl2���ƣ��ж����ж�����������ȶ���S2C12��������װ���Ʊ���
�ش��������⣺
��1��д��S2Cl2�ĵ���ʽ
 ��װ��a�е��Լ���Ũ���ᣮ
��װ��a�е��Լ���Ũ���ᣮ��2��д��ʵ������ȡCl2�����ӷ�Ӧ����ʽMnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��3��װ��b�з�����Ӧǰ������еIJ�������ͨ��Cl2���ž�װ���еĿ�����
��4��װ��e�����Ƿ�ֹ�����е�ˮ��������dʹ��Ʒ��ˮ��Ӧ������ʣ���Cl2��
��5��Ϊ�˴Ӳ�Ʒ���ᴿS2C12����Ҫ���еIJ��������Ƿ�������
��6����S2C12����ˮ�л�������������壬д��S2C12��ˮ��Ӧ�Ļ�ѧ����ʽ��2S2Cl2+2H2O=SO2��+3S��+4HCl������������������ķ����ǰ�����ͨ��Ʒ����Һ�У���ɫ���ټ����ָֻ���ɫ��֤����������SO2��
���� ��ʼ����b֮ǰ��ͨ����ˮ������ʼ���ɵ�S2Cl2������ȴҺ����S2Cl2��ˮˮ�⣬����ͨ��aƿ��������к���ˮ������ͨCl2һ��ʱ���������ɫ�������װ�ú��ž�װ���еĿ������ٿ�ʼ����b������۵�ϵͣ����������ۻ���װ��bˮƽ���÷�ֹ���ڵ���������ܣ�CΪ�����ܣ�ʹ���ɵ�S2Cl2������Һ�壬�����ռ���d������ƿ����e�����ü�ʯ�Ҿ�����ˮ���ã�����ˮ��Ӧ�����������ƣ���ֹ�����е�ˮ��������d�������ֹͣ���Ⱥ�ֹͣͨ������ƽ��������ѹǿ����ֹ����Σ�գ��ݴ��Ʊ�S2Cl2���ݴ˷������
��� �⣺��1��S2Cl2���ӽṹ��H2O2���ƣ�S2Cl2��������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ���ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�S2Cl2����ʽΪ�� ������Ϣ��֪S2Cl2��ˮˮ�⣬����b�е�����Ӧ�ø����Ũ������a��Ӧ���Լ�ΪŨ���ᣬ������Ϊ����������
������Ϣ��֪S2Cl2��ˮˮ�⣬����b�е�����Ӧ�ø����Ũ������a��Ӧ���Լ�ΪŨ���ᣬ������Ϊ����������
�ʴ�Ϊ�� ��Ũ���
��Ũ���
��2��ʵ����ͭ����������Ũ������ȡCl2�����ӷ���ʽΪ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
�ʴ�Ϊ��MnO2+4H++2Cl-$\frac{\underline{\;\;��\;\;}}{\;}$Mn2++Cl2��+2H2O��
��3��S2Cl2��������ˮ��Ӧ�������к���ˮ���������÷�Ӧ�������ž�װ���еĿ���������Ϊ����ɫ���壬����ͨCl2���ž�װ���еĿ������ٿ�ʼ����b��
�ʴ�Ϊ����ͨ��Cl2���ž�װ���еĿ�����
��4��װ��eʢ�ż�ʯ�ң���ֹ�����е�ˮ��������dʹ��Ʒ��ˮ��Ӧ������ʣ���Cl2��
�ʴ�Ϊ����ֹ�����е�ˮ��������dʹ��Ʒ��ˮ��Ӧ������ʣ���Cl2��
��5������е����ϴ�Ļ�����÷��������������¶ȡ�ˮ������������S��SCl2�����ʣ���Ҫ���еIJ����Ƿ��������õ�������S2Cl2��
�ʴ�Ϊ����������
��6����S2Cl2����ˮ�У��ڷ�Ӧ��������Ԫ��һ�������ߵ�+4�ۣ�����SO2����һ���ֽ��͵�0�ۣ�����S��������ԭ���غ��֪������ˮ����Ӧ����ʽΪ��2S2Cl2+2H2O=SO2��+3S��+4HCl�������������Ư���ԣ��ʼ����䷽��Ϊ��������ͨ��Ʒ����Һ�У���ɫ���ټ����ָֻ���ɫ��֤����������SO2��
�ʴ�Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl��������ͨ��Ʒ����Һ�У���ɫ���ټ����ָֻ���ɫ��֤����������SO2��
���� ���⿼�����ʵ��Ʊ������ؿ����ʵ��ԭ����װ�õ����⡢���ۣ��Ķ���Ŀ��ȡ��Ϣ�������ȣ��ؼ������������Ʊ�����ԭ�������������и�װ�õ����ã�Ҫ��ѧ��Ҫ����ʵ��ʵ�����֪ʶ�����Ӧ����Ϣ����������������Ŀ�Ѷ��еȣ�

| A�� | ������γɵ����ʽṹ��DNA˫�����ṹ����ؼ����� | |
| B�� | ԭ�ӹ������ڲⶨ������Ԫ�ص����� | |
| C�� | a����ɢ��ʵ�鼰�����˶��ķ��ֶ���ԭ��ģ�ͽ��������˹��� | |
| D�� | X-��������ʵ���������������ͷǾ��� |
| A�� | ���ȡҺ�м���BaCl2�а�ɫ����������˵�����к���SO42- | |
| B�� | ���ȡҺ�м���AgNO3��Һ�а�ɫ������˵�����к���Cl- | |
| C�� | �ྻ��˿պȡ��ȡҺ�������ھƾ��ƻ��������գ�����ʻ�ɫ������ȷ���Ƿ�K+ | |
| D�� | ���ȡҺ�еμ�ϡ���ᣬ������ɫ��ζ�����壬˵�����к���CO32- |
| ���ۼ� | ���� | ���ۼ� | ���� |
| H-H | 436 | H-F | 565 |
| C-F | 427 | H-S | 339 |
| C-C1 | 330 | H-Se | 314 |
| A�� | H2��g����2H��g����H=+436kJ/mol | |
| B�� | ����Խ�̣����ۼ�Խ�ι� | |
| C�� | ��ͬ������CH3F��CH3C1������ˮ�ⷴӦ | |
| D�� | ��ͬѹǿ�£�H2S�ķе��H2Se�ķе�� |

��1��ͭ��Ũ���ᷴӦ�����ӷ���ʽΪCu+4H++2NO3-=Cu2++2NO2��+2H2O������Ũ�����װ�â�����ƿ�ϲ��ɹ۲쵽���������к���ɫ�������ɣ�
��2������a������Ϊ���θ���ܣ���������ʢװ���Լ���D�����ţ���
A����ʯ��B��Ũ����C����ʯ��D������������
��3����֪�������ʵ��й����ݣ�
| ���� | �۵�/�� | �е�/�� |
| N2O5 | 41 | 32 �������� |
| N2O4 | -11 | 24 |
��4��װ�â�������ǰ�ȫƿ����ֹ������
��5����ѧ��ȤС�����Ƶõ�N2O5�Ʊ������������ױ���
 ����Է�������137�����������£���������ƿ�з��������30mL N2O5��CH2Cl2��Һ��N2O5��Ũ��Ϊlmol•L-1����30��ʱ���μ�15mL�ױ�����ַ�Ӧ�ö������ױ� 1.73g���ش��������⣺
����Է�������137�����������£���������ƿ�з��������30mL N2O5��CH2Cl2��Һ��N2O5��Ũ��Ϊlmol•L-1����30��ʱ���μ�15mL�ױ�����ַ�Ӧ�ö������ױ� 1.73g���ش��������⣺���Ʊ��������ױ��Ļ�ѧ����ʽΪ
 +N2O5$��_{��}^{����}$
+N2O5$��_{��}^{����}$ +H2O��
+H2O����N2O5���ɶ������ױ���ת����Ϊ21.05%��
��1��һ�����¶Ⱥʹ��������£����ü��顢����Ϊԭ�������ϳ������ᣮ
�ٰ����ĵ���ʽΪ
 ��
���ںϳ�������Ļ�ѧ����ʽΪ2CH4+2NH3+3O2$\frac{\underline{\;һ������\;}}{����}$2HCN+6H2O
��2����֪25��ʱHCN��H2CO3�ĵ��볣����Ka�������
| ���� | ���볣����Ka�� |
| HCN | Ka=5��10-10 |
| H2CO3 | Ka1=4.5��10-7 Ka2=4.7��10-11 |
��3��-�������£�HCN��H2��H2O��Ӧ���£�
I��HCN��g��+3H2��g��?NH3��g��+CH4��g����H1
��HCN��g��+H2O��g��?NH3��g��+CO��g����H2
�ٷ�Ӧ��CO��g��+3H2��g��?CH4��g��+H2O��g���ġ�H=��H1-��H2 ���á�H1����H2��ʾ����
�ڶ��ڷ�Ӧ��Сѹǿ��HCN��ת���ʲ��䣨���ߡ��������䡱���͡�����
�۷�ӦI�����ƽ�ⳣ������ֵ��lgK�����¶ȵĹ�ϵ��ͼ����ʾ����T1Kʱ����Ӧ���ƽ�ⳣ������ֵlgK=10��

��4����ⷨ���������ˮ��ԭ����ͼ����ʾ������CN-�ȷ����缫��Ӧ��CN-+2OH--2e-�TCNO-+H2O��CNO-�������Ͻ�һ�������ĵ缫��ӦʽΪ2CNO-+4OH--6e-=N2��+2CO2��+2H2O��

 ������װ�ú��װ�������Եķ����Ǽ��װ�������Եķ����ǣ��ر�A�з�Һ©���Ļ�������E�м�������ˮ����A�е�Բ����ƿ��E��������ð������ȴ��E�в������γ�һ��ˮ���������������ã�
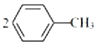
������װ�ú��װ�������Եķ����Ǽ��װ�������Եķ����ǣ��ر�A�з�Һ©���Ļ�������E�м�������ˮ����A�е�Բ����ƿ��E��������ð������ȴ��E�в������γ�һ��ˮ���������������ã� ��1��ijͬѧ�����ͼ1װ����ȡ�����屽��д��ʵ������ȡ�屽�Ļ�ѧ����ʽ
��1��ijͬѧ�����ͼ1װ����ȡ�����屽��д��ʵ������ȡ�屽�Ļ�ѧ����ʽ ��
��
