��Ŀ����
Ϊ�ⶨij±��������ɣ�ijͬѧ���ʵ�����£��ٽ������������أ�����ȴ����ϡ�����ữ���ټ���������������۹��˳�����ϴ�ӳ���2��3�Σ�����ȡ��±����Һ��10.0mL����������NaOH��Һ�����ȷ�Ӧ��Һ�岻�ֲ㣻�ش��������⣺
��1���밴����ȷ�IJ����������� ��������Żش�
��2�����м���ϡ�����Ŀ���� �����м��������������Ŀ���� ��
��3�����������ɵij���Ϊ����ɫ�����±�����е�±ԭ���� ��
��4�����Ƶó���������Ϊ37.6g���ֲ��±�������ܶ�Ϊ1.88g?mL-1���������ܶ�����ͬ�����������ܶȵ�94�������±���������к��� ��±ԭ�ӣ�
��1���밴����ȷ�IJ�����������
��2�����м���ϡ�����Ŀ����
��3�����������ɵij���Ϊ����ɫ�����±�����е�±ԭ����
��4�����Ƶó���������Ϊ37.6g���ֲ��±�������ܶ�Ϊ1.88g?mL-1���������ܶ�����ͬ�����������ܶȵ�94�������±���������к���
���㣺±�������,̽�����ʵ���ɻ�������ʵĺ���
ר�⣺�л���������ͨʽ��Ӧ�ù���
��������1�����ݼ���±��������ȷ����������������
��2������±������ˮ����Ҫ�ڼ��������½��У�����±��ԭ��ǰ�����к�������н�𣻼����������������ȷ��±��ԭ����ȫת���ɳ�����
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr��
��4������n=
����AgBr�����ʵ��������л���������ܶ�����ͬ�����������ܶȵ�94�����������Է�������������m=��V������л�����������ٸ���n=
���±���������ʵ�����ȷ��±�����е�±ԭ����Ŀ��
��2������±������ˮ����Ҫ�ڼ��������½��У�����±��ԭ��ǰ�����к�������н�𣻼����������������ȷ��±��ԭ����ȫת���ɳ�����
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr��
��4������n=
| m |
| M |
| m |
| M |
���
�⣺��1������±��ԭ�ӵķ���Ϊ����±����������������Һ�Ļ��Һ���ȡ���ȴ�����������Һ�к����������ơ�������������Һ���顢�������ɵ�±��������������������ȷ����˳��Ϊ���ܢڢۢ٣�
�ʴ�Ϊ���ܢڢۢ٣�
��2��±������ˮ����Ҫ�ڼ�����Һ�н��У�������������Һ����±��ԭ��ǰ��Ҫ�ȼ���ϡ�����к���������������Һ�������������������Ŀ���ǽ����е�±��ԭ����ȫת���ɳ�����ʹ���������ȷ��
�ʴ�Ϊ���к���������������Һ��ʹ±���ӳ�����ȫ��
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr�����Ը�±�����е�±ԭ����Br��
�ʴ�Ϊ��Br��
��4�����Ƶó���������Ϊ37.6g��n��AgBr��=
=0.2mol��
��֪���л���������ܶ�����ͬ�����������ܶȵ�94����������Է�������Ϊ188��m=��V=1.65g?mL-1��11.4mL=18.8g�����л�������ʵ���n=
=0.1mol��Br�����ʵ�����±���������ʵ���֮��Ϊ2��1������±�����е�Brԭ����ĿΪ2��
�ʴ�Ϊ��2��
�ʴ�Ϊ���ܢڢۢ٣�
��2��±������ˮ����Ҫ�ڼ�����Һ�н��У�������������Һ����±��ԭ��ǰ��Ҫ�ȼ���ϡ�����к���������������Һ�������������������Ŀ���ǽ����е�±��ԭ����ȫת���ɳ�����ʹ���������ȷ��
�ʴ�Ϊ���к���������������Һ��ʹ±���ӳ�����ȫ��
��3�����������ɵij���Ϊ����ɫ����ó���ΪAgBr�����Ը�±�����е�±ԭ����Br��
�ʴ�Ϊ��Br��
��4�����Ƶó���������Ϊ37.6g��n��AgBr��=
| 37.6g |
| 188g/mol |
��֪���л���������ܶ�����ͬ�����������ܶȵ�94����������Է�������Ϊ188��m=��V=1.65g?mL-1��11.4mL=18.8g�����л�������ʵ���n=
| 18.8g |
| 188g/mol |
�ʴ�Ϊ��2��
���������⿼����±������±��ԭ�ӵļ��鷽������Ŀ�ѶȽϴ�ע�����ռ���±������±��ԭ�ӵļ��鷽��������������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д�
�����Ŀ
�������ʲ���ʹ����KMnO4��Һ��ɫ���ǣ�������
| A��C4H10 |
| B��C2H2 |
C��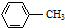 |
| D��CH3COOH |
���е�ع���ʱ��O2�������ŵ���ǣ�������
A�� п�̵�� |
B�� ��ȼ�ϵ�� |
C�� Ǧ���� |
D�� ���ӵ�� |
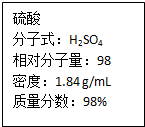 ��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺
��ͼΪʵ����ijŨ�����Լ�ƿ�ϵı�ǩ���Ը����й����ݻش��������⣺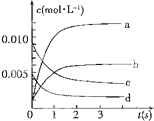 ��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������ڣ�800��ʱ��Ӧ��2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯����� �����ѣ�CH3OCH3������Ϊ21���������ȼ�ϣ���δ������������͡�Һ������ú���Ȳ����������Ļ������ܣ���ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2.0��10.0Mpa���¶�230��280�棩�������з�Ӧ��
�����ѣ�CH3OCH3������Ϊ21���������ȼ�ϣ���δ������������͡�Һ������ú���Ȳ����������Ļ������ܣ���ҵ�Ʊ��������ڴ���Ӧ���У�ѹ��2.0��10.0Mpa���¶�230��280�棩�������з�Ӧ��