��Ŀ����
18��ij��ɫ��Һ�ֻ�����������ӣ�Mg2+��H+��Ag+��Na+��Cl-��HCO-3��OH-��NO-3�еļ��֣���֪������Һ�ܺͽ�������Ӧ���ų�������ֻ���������Իش���1������Һ������Ӧ����AlO-2����ԭ��Һһ�����е�������Na+��OH-��������Ӧ�����ӷ���ʽ��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2������Һ������Ӧ����Al3+���ɣ���ԭ��Һ��һ�������е�������Ag+��OH-��HCO3-��NO3-��
���� ����������Ӧ����H2����Һ����Ϊ����Һ��Ҳ����Ϊ����Һ��������Al3+ʱ����ҺΪ����Һ��������AlO2-ʱ����ҺΪ����Һ��Ȼ���������ӵĹ�������������ע��������ˮ�е���ɫ��
��� �⣺���Ӿ�Ϊ��ɫ���������Ӿ�����ɫ����Һ���ϣ��������������ò���������Ҳ��������ò���������
��1����Һ�����۷�Ӧ����AlO2-���ɣ�2Al+2NaOH+2H2O=2NaAlO2+3H2������Һ�Լ���ʱ��Mg2+��H+��Ag+��HCO3-���ܴ��ڣ�������Һ���Ե��ԣ�һ�����������ӣ���������ֻ��Na+��˵��ԭ��Һ�е�������һ����OH-�����ܺ�NO3-��Cl-����Ӧ�Ļ�ѧ����ʽΪ���ʴ�Ϊ��Na+��OH-��2Al+2NaOH+2H2O=2NaAlO2+3H2����
��2����Һ�����۷�Ӧ����Al3+���ɣ���Һ�����ԣ���HCO3-��OH-�����ڣ����������������Ӧһ��û���������������Ҳ������NO3-��������Һ���Ե��ԣ�һ�����������ӣ�����Һ�п϶���Cl-����Ag+����Cl-���ɳ�����˵��ԭ��Һ��Ҳ������Ag+������Һ��һ�����д�����H+��Cl-�����ܺ�Na+��Mg2+��һ�����ܺ��У�Ag+��OH-��HCO3-��NO3-���ʴ�Ϊ��Ag+��OH-��HCO3-��NO3-��
���� ���⿼�����ʵļ��鼰���ӵĹ������⣬��ȷ��������֮��ķ�Ӧ�ǽ����Ĺؼ�����ע��������ҺΪ�����ԡ���Һ������Ե���������ɣ���Ŀ�ѶȲ���

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�| A�� | ������Ȳ���Ƿ����H2S��ʪ��Ĵ���Ǧ��ֽ | |
| B�� | ����ζ�NaHCO3��Һ����̪ | |
| C�� | ��������Ƿ���ȫˮ�⣺��ˮ | |
| D�� | �����Ȼ����Ƿ�����ʪ���pH��ֽ |
| A�� | I2�������� | B�� | ƽ�������ƶ� | C�� | ���������������棩 | D�� | HIת���ʲ��� |
| A�� | NaCl | B�� | NaOH | C�� | HCl | D�� | Cl2 |
| A�� | 0.09mol | B�� | 0.11mol | C�� | 0.12mol | D�� | 0.2mol |
| ѡ�� | n��CO2��/mol | ��Һ�����ӵ����ʵ���Ũ�� |
| A | 0 | c��Na+����c��AlO2-��+c��OH-�� |
| B | 0.01 | c��Na+����c��AlO2-����c��OH-����c��CO32-�� |
| C | 0.015 | c��Na+����c��HCO3-����c��CO32-����c��OH-�� |
| D | 0.03 | c��Na+����c��HCO3-����c��OH-����c��H+�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
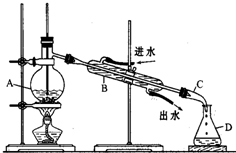 ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺
ijͬѧ���������װ�ý���ʯ�������ʵ�飬�ش������й����⣺