МвДҝДЪИЭ
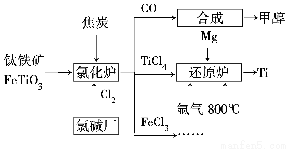
ОӘБЛМбёЯЧКФҙАыУГВКЈ¬јхЙЩ»·ҫіОЫИҫЈ¬»Ҝ№ӨјҜНЕҪ«оСі§ЎўВИјоі§әНјЧҙјі§ЧйіЙІъТөБҙЈ¬ИзНјЛщКҫЎЈ
ЗлМоРҙПВБРҝХ°ЧЎЈ
(1)оСМъҝуҪшИлВИ»ҜВҜЗ°НЁіЈІЙИЎПҙөУЎў·ЫЛйЎўәжёЙЎўФӨИИөИОпАн·Ҫ·ЁҙҰАнЈ¬ЗлҙУФӯАнЙПҪвКН·ЫЛйөДЧчУГЈә_______________________________________
ТСЦӘВИ»ҜВҜЦРВИЖшәНҪ№МҝөДАнВЫУГБПОпЦКөДБҝЦ®ұИОӘ7ЎГ6Ј¬ФтВИ»ҜВҜЦР»№ФӯјБөД»ҜС§КҪКЗ___________________________ЎЈ
(2)ТСЦӘЈәўЩMg(s)Ј«Cl2(g)=MgCl2(s)ҰӨHЈҪЈӯ641 kJ/mol
ўЪ2Mg(s)Ј«TiCl4(s)= 2MgCl(s)Ј«Ti(s)ҰӨHЈҪЈӯ512 kJ/mol
ФтTi(s)Ј«2Cl2(g)=TiCl4(s)ЎЎҰӨHЈҪ________ЎЈ
(3)лІЖшНЁИл»№ФӯВҜЦРІўІ»ІОУл·ҙУҰЈ¬НЁИллІЖшөДЧчУГКЗ___________________________
(4)ТФјЧҙјЎўҝХЖшЎўЗвСх»ҜјШИЬТәОӘФӯБПЈ¬КҜД«ОӘөзј«ҝЙ№№іЙИјБПөзіШЎЈТСЦӘёГИјБПөзіШөДЧЬ·ҙУҰКҪОӘ2CH3OHЈ«3O2Ј«4OHЈӯ=2CO32ЎӘЈ«6H2OЈ¬ёГөзіШЦРХэј«ЙПөДөзј«·ҙУҰКҪОӘ_________________________________________ЎЈ
№ӨЧчТ»¶ОКұјдәуЈ¬ІвөГИЬТәөДpH________(МоЎ°јхРЎЎұЎўЎ°ФцҙуЎұ»тЎ°І»ұдЎұ)ЎЈ
(1)Фцҙу·ҙУҰОпјдөДҪУҙҘГж»эЈ¬МбёЯ·ҙУҰЛЩВКЎЎCЎўFeTiO3
(2)Јӯ770 kJ/mol
(3)MgәНTi¶јУРЗҝ»№ФӯРФЈ¬ФЪлІЖш·ХО§ЦРҝЙ·АЦ№MgЎўTiұ»Сх»Ҝ
(4)O2Ј«2H2OЈ«4eЈӯ=4OHЈӯ(»т3O2Ј«6H2OЈ«12eЈӯ=12OHЈӯ)ЎЎјхРЎ
ЎҫҪвОцЎҝ(1)·ЫЛй·ҙУҰОпЈ¬ҝЙТФФцҙуЖдұнГж»эЈ¬ҙУ¶шФцҙу·ҙУҰОпЦ®јдөДҪУҙҘГж»эЈ¬МбёЯ·ҙУҰЛЩВКЈ»ВИ»ҜВҜЦР·ўЙъөД·ҙУҰОӘ6CЈ«7Cl2Ј«2FeTiO3=6COЈ«2TiCl4Ј«2FeCl3Ј¬УЙҙЛҝЙЦӘCәНFeTiO3ОӘ»№ФӯјБЎЈ(2)ёщҫЭёЗЛ№¶ЁВЙЈ¬УЙўЩЎБ2ЈӯўЪөГЈәTi(s)Ј«2Cl2(g)=TiCl4(s)Ј»ҰӨHЈҪЈӯ770 kJ/molЎЈ(3)лІЖшРФЦКІ»»оЖГҝЙТФ·АЦ№MgәНTiөИҫЯУРЗҝ»№ФӯРФөДОпЦКұ»Сх»ҜЎЈ(4)ёщҫЭёГөзіШөДЧЬ·ҙУҰКҪҝЙЦӘЈ¬·ҙУҰ№эіМЦРІ»¶ППыәДOHЈӯЈ¬ФтёГөзіШ№ӨЧчТ»¶ОКұјдәуЈ¬ИЬТәөДpHҪ«јхРЎЎЈ

SiO2ЎўSO2әНCO2¶јКЗЛбРФСх»ҜОпЈ¬ЛьГЗөД»ҜС§РФЦКҫЯУРТ»¶ЁөДПаЛЖРФЈ»MgәНNaөД»ҜС§РФЦКТІҫЯУРТ»¶ЁПаЛЖРФЎЈ
ДіРЛИӨРЎЧйУГИзНјЛщКҫЧ°ЦГҪшРРMgУлSO2·ҙУҰөДКөСйЎЈ

(1)СЎФсЦЖИЎSO2өДәПКККФјБ________(МоұаәЕ)ЎЈ
ўЩЕЁHClЎЎўЪЕЁH2SO4ЎЎўЫNa2SO3№ММеЎЎўЬCaSO3№ММе
(2)ЙПКцЧ°ЦГ»№ҝЙУЕ»ҜЈ¬УЕ»ҜөД·Ҫ·ЁКЗ________________________________________Ј¬Ч°ЦГCЦРNaOHИЬТәөДЧчУГКЗ___________________________________________________________
(3)јЧН¬С§НЖІвMgУлSO2өД·ҙУҰәНMgУлCO2өД·ҙУҰПаЛЖЈ¬ФтёГ·ҙУҰ·ҪіМКҪОӘ_________________________________________Ј»
ТТН¬С§өДНЖІвКЗЈә2MgЈ«3SO2 2MgSO3Ј«SЈ»ұыН¬С§өДНЖІвКЗЈә3MgЈ«SO2
2MgSO3Ј«SЈ»ұыН¬С§өДНЖІвКЗЈә3MgЈ«SO2 2MgOЈ«MgSЈ¬ТӘСйЦӨјЧЎўТТЎўұыИэО»Н¬С§өДНЖІвКЗ·сХэИ·Ј¬¶ЎН¬С§ЧчИзПВКөСйМҪҫҝЈә
2MgOЈ«MgSЈ¬ТӘСйЦӨјЧЎўТТЎўұыИэО»Н¬С§өДНЖІвКЗ·сХэИ·Ј¬¶ЎН¬С§ЧчИзПВКөСйМҪҫҝЈә
ТСЦӘЈәMgSO3әНMgS¶јОўИЬУЪЛ®Ј¬ДЬУлСОЛб·ўЙъёҙ·ЦҪв·ҙУҰ·ЕіцЖшМеЈ»H2SЖшМеНЁИлCuSO4ИЬТәЦРіцПЦәЪЙ«іБөнЎЈ
ПЮСЎКФјБЈә2 molЎӨLЈӯ1СОЛбЎў2 molЎӨLЈӯ1ПхЛбЎўХфБуЛ®Ўў2 molЎӨLЈӯ1 NaOHИЬТәЎўЖ·әмИЬТәЎўіОЗеКҜ»ТЛ®Ўў2 molЎӨLЈӯ1 CuSO4ИЬТәЈ»ТЗЖчәНУГЖ·ЧФСЎЎЈ
РтәЕ | КөСйІҪЦи | ФӨЖЪПЦПуәНҪбВЫ |
ўЩ | ИЎЙЩБҝ·ҙУҰәуЛщөГ№ММеУЪКФ№ЬЦР |
|
ўЪ | ПтКФ№ЬЦРөД№ММеВэВэөОјУ____________Ј¬КФ№ЬҝЪИыЙПҙшөј№ЬөДөҘҝЧИыЈ¬ІўҪ«өј№ЬНЁИлКўУР________өДКФ№ЬЦР | ИфКФ№ЬЦРөД________Ј¬ФтұыН¬С§НЖІвХэИ·Ј¬ИфКФ№ЬЦРөД№ММеОҙНкИ«ИЬҪвЈ¬ЗТ________Ј¬ФтТТН¬С§НЖІвХэИ· |
ёщҫЭЙПКцКөСйМҪҫҝЈ¬ДЬЦӨГчјЧН¬С§НЖІвХэИ·өДІЩЧчәНФӨЖЪПЦПуКЗ
_____________________________________________________________ЎЈ
(4)ЙПКцКөСйРиТӘ100 mL 2 molЎӨLЈӯ1өДСОЛбЈ¬ЕдЦЖКұСЎУГ________(СЎМо10 mLЎў25 mLЎў50 mL»т100 mL)БҝНІБҝИЎ36.5%ГЬ¶ИОӘ1.19 gЎӨmLЈӯ1өДЕЁСОЛбөДМе»эОӘ________mLЎЈ
1 LДі»мәПИЬТәЈ¬ҝЙДЬә¬УРөДАлЧУИзПВұнЎЈ
ҝЙДЬҙуБҝә¬УРөДСфАлЧУ | HЈ«ЎўKЈ«ЎўMg2Ј«ЎўAl3Ј«ЎўNH4+ЎўFe2Ј«ЎўFe3Ј« |
ҝЙДЬҙуБҝә¬УРөДТхАлЧУ | ClЈӯЎўBrЈӯЎўIЈӯЎўCO32ЎӘЎўAlO2ЎӘ |
(1)НщёГИЬТәЦРЦрөОјУИлNaOHИЬТәЈ¬ІъЙъіБөнөДОпЦКөДБҝ(n)УлјУИлNaOHИЬТәөДМе»э(V)өД№ШПөИзНјЛщКҫЎЈФтёГИЬТәЦРТ»¶ЁІ»ә¬УРөДАлЧУКЗ_________________________ЎЈ

(2)BC¶ОАлЧУ·ҪіМКҪОӘ_______________________________________________ЎЈ
(3)V1ЎўV2ЎўV3ЎўV4Ц®јдөД№ШПөОӘ__________________________________________ЎЈ
(4)ҫӯјмІвЈ¬ёГИЬТәЦР»№ә¬УРҙуБҝөДClЈӯЎўBrЈӯЎўIЈӯЈ¬ИфПт1 LёГ»мәПИЬТәЦРНЁИлТ»¶ЁБҝөДCl2Ј¬ИЬТәЦРClЈӯЎўBrЈӯЎўIЈӯөДОпЦКөДБҝУлНЁИлCl2өДМе»э(ұкЧјЧҙҝц)өД№ШПөИзұнЛщКҫЈ¬·ЦОцәу»ШҙрПВБРОКМвЎЈ
Cl2өДМе»э(ұкЧјЧҙҝц) | 2.8 L | 5.6 L | 11.2 L |
n(ClЈӯ) | 1.25 mol | 1.5 mol | 2 mol |
n(BrЈӯ) | 1.5 mol | 1.4 mol | 0.9 mol |
n(IЈӯ) | a mol | 0 | 0 |
ўЩөұНЁИлCl2өДМе»эОӘ2.8 LКұЈ¬ИЬТәЦР·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪОӘ_________________ЎЈ
ўЪФӯИЬТәЦРClЈӯЎўBrЈӯЎўIЈӯөДОпЦКөДБҝЕЁ¶ИЦ®ұИОӘ_____________________________ЎЈ
№ӨТөМјЛбДЖ(ҙҝ¶ИФјОӘ98%)ЦРә¬УРCa2Ј«ЎўMg2Ј«ЎўFe3Ј«ЎўClЈӯәНSO42ЎӘөИФУЦКЈ¬Мбҙҝ№ӨТХБчіМИзПВЈә

ўс.МјЛбДЖөДұҘәНИЬТәФЪІ»Н¬ОВ¶ИПВОціцөДИЬЦКИзПВНјЛщКҫЈә

ўт.УР№ШОпЦКөДИЬ¶И»эИзПВЈә
ОпЦК | CaCO3 | MgCO3 | Ca(OH)2 | Mg(OH)2 | Fe(OH)3 |
Ksp | 4.96ЎБ10Јӯ9 | 6.82ЎБ10Јӯ6 | 4.68ЎБ10Јӯ6 | 5.61ЎБ10Јӯ12 | 2.64ЎБ10Јӯ39 |
»ШҙрПВБРОКМвЈә
(1)јУИлNaOHИЬТәәу№эВЛөГөҪөДВЛФьЦРЦчТӘә¬УР________(МоРҙ»ҜС§КҪ)ЎЈ25ЎжКұЈ¬Птә¬УРMg2Ј«ЎўFe3Ј«өДИЬТәЦРөОјУNaOHИЬТәЈ¬өұБҪЦЦіБөн№ІҙжЗТИЬТәөДpHЈҪ8 КұЈ¬c(Mg2Ј«)ЎГc(Fe3Ј«)ЈҪ________ЎЈ
(2)ІЩЧчXОӘ________Ј¬ЖдОВ¶ИУҰҝШЦЖФЪ_____________________________________
(3)УРИЛҙУЎ°ВМЙ«»ҜС§ЎұҪЗ¶ИЙиПлҪ«Ў°ДёТәЎұСШБчіМЦРРйПЯЛщКҫҪшРРСӯ»·К№УГЎЈЗлДг·ЦОцКөјК№ӨТөЙъІъЦРКЗ·сҝЙРР________Ј¬ІўЛөГчАнУЙ______________________________
________________________________________________________________________ЎЈ




