��Ŀ����
17�������ṩ�����ƶ�Ԫ�أ�����Ҫ����գ���1��ԭ�Ӻ�����3�����Ӳ㣬��۵�����Ϊ7������������Ӧˮ���ﻯѧʽHClO4���䵥����NaOH��Ӧ�Ļ�ѧ����ʽΪCl2+2NaOH�TNaClO+NaCl+H2O��
��2����֪X+��Y2+��Z-��W2-�������Ӿ�������ͬ�ĵ��Ӳ�ṹ����X��Y��Z��W������Ԫ�ص�ԭ�������ɴ�С��˳����Y��X��Z��W��ԭ�Ӱ뾶�ɴ�С��˳����X��Y��W��Z��
��3��A+��B-��C��D �������ӣ����ӻ����ӣ������Ƕ��ֱ�10�����ӣ���֪����������ת����ϵ��A++B-$\stackrel{��}{��}$C+D��������A+��B-�ĵ���ʽ
 ���Ƚ�C��D���ȶ��Ե�ǿ����СH2O��NH3���û�ѧʽ��ʾ��
���Ƚ�C��D���ȶ��Ե�ǿ����СH2O��NH3���û�ѧʽ��ʾ����4����NH4NO3�� ��NaF�� ��CO2�� ��K2O2�� ��NaOH ��CH4
ֻ���м��Լ����Ǣۢޣ��������Ӽ����зǼ��Լ����Ǣܣ��������Ӽ����м��Լ����Ǣ٢ݣ�
���� ��1��ԭ�Ӻ�����3�����Ӳ㣬��۵�����Ϊ7��ΪClԪ�أ�����������Ӧˮ����Ϊ�����ᣬ������NaOH��Ӧ����NaCl��NaClO��ˮ��
��2��������ͬ�ĵ��Ӳ�ṹ�����ӣ���ǰ���£��������������棻���Ӳ�Խ�࣬ԭ�Ӱ뾶Խ��ͬ����ԭ���������ԭ�Ӱ뾶С��
��3�����ֱ�10�����ӣ���A++B-$\stackrel{��}{��}$C+D����֪��笠����������������ӷ�Ӧ���ɰ�����ˮ���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ���
��4��һ����˵�����ý�����ǽ����γ����Ӽ����ǽ���֮���γɹ��ۼ�����ͬ�ǽ����γɼ��Թ��ۼ���ͬ�ַǽ����γɷǼ��Լ����Դ������
��� �⣺��1��ԭ�Ӻ�����3�����Ӳ㣬��۵�����Ϊ7��ΪClԪ�أ�����������Ӧˮ����Ϊ�����ᣬ�仯ѧʽΪHClO4��������NaOH�ķ�ӦΪCl2+2NaOH�TNaClO+NaCl+H2O��
�ʴ�Ϊ��HClO4��Cl2+2NaOH�TNaClO+NaCl+H2O��
��2��������ͬ�ĵ��Ӳ�ṹ�����ӣ���ǰ���£�ԭ������������Ӱ뾶С����X��Y��Z��W������Ԫ�ص�ԭ�������ɴ�С��˳����Y��X��Z��W��ԭ�Ӱ뾶�ɴ�С��˳����X��Y��W��Z��
�ʴ�Ϊ��Y��X��Z��W��X��Y��W��Z��
��3�����ֱ�10�����ӣ���A++B-$\stackrel{��}{��}$C+D����֪��笠����������������ӷ�Ӧ���ɰ�����ˮ����A+��B-�ĵ���ʽ�ֱ�Ϊ ���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����C��D���ȶ��Ե�ǿ����СΪH2O��NH3��
���ǽ�����Խǿ����Ӧ�⻯��Խ�ȶ�����C��D���ȶ��Ե�ǿ����СΪH2O��NH3��
�ʴ�Ϊ�� ��H2O��NH3��
��H2O��NH3��
��4����NH4NO3�к����Ӽ���N-H��N-O���Լ���
��NaFֻ�����Ӽ���
��CO2��ֻ�����Թ��ۼ���
��K2O2�к����Ӽ���O-O�Ǽ��Լ���
��NaOH�к����Ӽ���O-H���Լ���
��CH4��ֻ�����Թ��ۼ���
ֻ���м��Լ����Ǣۢޣ��������Ӽ����зǼ��Լ����Ǣܣ��������Ӽ����м��Լ����Ǣ٢ݣ�
�ʴ�Ϊ���ۢޣ��ܣ��٢ݣ�
���� ���⿼��ԭ�ӽṹ�������ɡ���ѧ���ȣ�Ϊ��Ƶ���㣬����ԭ�ӽṹ��Ԫ�������ɡ���ѧ�����γɼ��ж�Ϊ���Ĺؼ������ط�����Ӧ�������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�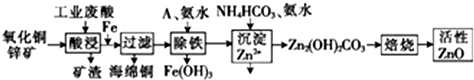
��֪�������ӿ�ʼ��������ȫ����ʱ��pH���±���ʾ��
| ���� | ��ʼ����ʱ��pH | ��ȫ����ʱ��pH |
| Fe2+ | 6.34 | 9.7 |
| Fe3+ | 1.48 | 3.7 |
| Zn2+ | 6.2 | 8.0 |
��1���ڡ�����������У�Ϊ��߽������ʣ���ͨ����������⣬���ɲ�ȡ�Ĵ�ʩ���ʵ������¶ȣ���������Ũ�ȡ�������ͭп�����ȣ�
��2������ͭп���к���������CuS��ZnS����H2SO4��������ZnS�����ܽ��CuS���ܣ�����ͬ�¶��£�Ksp��CuS�������������������=����Ksp��ZnS����
��3������A���ʹ�����������е�B��
A��KMnO4���� B������������C��HNO3������D��NaClO
��4�����������м��백ˮ��Ŀ���ǵ�����Һ��pH��pHӦ������3.2��6.2��Χ֮�䣮
��5������B�ǿ�ֱ���������ʵ����Σ���B�Ļ�ѧʽ�ǣ�NH4��2SO4��
��6��������õ���Fe��OH��3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ������--K2FeO4��д���÷�Ӧ�����ӷ���ʽ��2Fe��OH��3+3ClO-+4OH-=2FeO42-+3Cl-+5H2O��
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
��2����ͬѧ�Ʋ����ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ�������𣺺��������������������������д�����ȷ�Ӧ��һ�ֹ�ҵ��;���Ӹֹ졢ұ�����۽����������ƣ�
��3�����һ����ʵ�鷽����֤���������õĿ�״�������к��н���������ʵ�������Լ���NaOH��Һ����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O=2AlO2-+3H2����
��4��ʵ����Ҫ�ܽ������������Լ��п���ѡ��BC������ţ���
A��Ũ���� B��ϡ����C��ϡ���� D������������Һ��
| A�� | x=4 | B�� | B��ת����Ϊ60% | ||
| C�� | A��ƽ��Ũ����2.8mol/L | D�� | ƽ��ʱ�����ѹǿ��ԭ����0.94�� |
| A�� | 1 mol H2SO4������Ϊ98g•mol-1 | |
| B�� | H2SO4��Ħ������Ϊ98g | |
| C�� | 9.8 g H2SO4����NA��H2SO4���� | |
| D�� | 6.02��1022��H2SO4���ӵ�����Ϊ9.8g |
| A�� | ��������W���淴Ӧ�������� | |
| B�� | ͨ��һ����������ѹǿ����ƽ��������Ӧ�����ƶ� | |
| C�� | �����¶ȣ�����Ӧ���������淴Ӧ���ʼ�С��ƽ��������Ӧ�����ƶ� | |
| D�� | �����¶ȣ�����Ӧ���ʼ�С���淴Ӧ����Ҳ��С��ƽ�����淴Ӧ�����ƶ� |
| A�� | ˮ��ɱ�������ͣ��ܶȱ�С | |
| B�� | 31g�������д��ڵĹ��ۼ���Ŀ��1.5NA��P�����ԭ������31�� | |
| C�� | DNA�еļ�����������ͨ�������ʵ�ֵ� | |
| D�� | H2O��һ�ַdz��ȶ��Ļ������������������� |

 ��Ԫ�ؿ����γ�HClO��HClO2��HClO3��HClO4���ֺ����ᣮ
��Ԫ�ؿ����γ�HClO��HClO2��HClO3��HClO4���ֺ����ᣮ