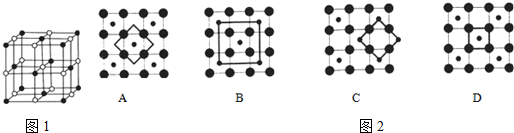
��Ŀ����
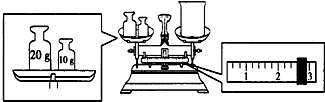
16��ʵ����Ҫ��NaOH��������100mL 2mol•L-1��NaOH��Һ���Իش����и��⣺��1�����������У�һ�������õ�����A
A����ƿ B����Ͳ C����ͷ�ι� D��100mL����ƿ E��������ƽ
��2����Ҫʵʩ���ƣ������������⣬��ȱ�IJ�����������Ʒ���ձ�����������
��3�����dz������ƹ��̼���Ϊ���¸����裺
A����ȴ B������ C��ϴ�� D������ E���ܽ� F��ҡ�� G��ת����Һ
����ȷ�IJ���˳��Ӧ��BEAGCDF�����������ţ���
��4�����в����ᵼ�������Ƶ���ҺŨ��ƫ�ߵ���C
A������ʱ��������ƿ�̶���
B������ƿ��������ˮ
C��δ��ȴ����
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ�
��5������������ƽ����NaOH8.0�ˣ�
���� ��1����2����������һ�����ʵ���Ũ����Һһ�㲽��ѡ����Ҫ������
��3������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��ݴ�����
��4���������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��5������m=CVM������Ҫ���ʵ�������
��� �⣺��1����2������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������Ҫ������������ƽ��Կ�ס��ձ�����Ͳ��������������ƿ����ͷ�ιܣ�����100mL 2mol•L-1��NaOH��Һ��Ӧѡ��100mL����ƿ�������ò�����������A��
��ȱ�ٵ��������ձ�����������
�ʴ�Ϊ����1��A����2���ձ�����������
��3������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ�������ȷ��˳��Ϊ��BEAGCDF��
�ʴ�Ϊ��BEAGCDF��
��4��A������ʱ��������ƿ�̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���A��ѡ��
B������ƿ��������ˮ�������ʵ����ʵ�������Һ������������Ӱ�죬��ҺŨ�Ȳ��䣬��B��ѡ��
C��δ��ȴ���ݣ���ȴ����Һ���ƫС����ҺŨ��ƫ�ߣ���Cѡ��
D�����ݺ�����ƿ����ҡ�Ⱥ���Һ����ڿ̶��ߣ��㲹�伸��ˮ���̶ȴ������µ�����Һ���ƫ����ҺŨ��ƫ�ͣ���D��ѡ��
��ѡ��C��
��5����NaOH��������100mL 2mol•L-1��NaOH��Һ����Ҫ������������m=0.1L��2mol/L��40g/mol=8.0g��
�ʴ�Ϊ��8.0��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������C=$\frac{n}{V}$�����������ķ����ͼ��ɣ���Ŀ�ѶȲ���

| A�� | 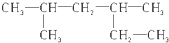 2��4-�������� 2��4-�������� | |
| B�� | 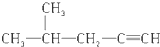 2-��-4-��Ȳ 2-��-4-��Ȳ | |
| C�� | 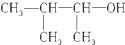 3-��-2-���� 3-��-2-���� | |
| D�� | CH3-CHBr-CHBr-CH3 2��3-���嶡�� |
| A�� | ��ʯ�� | B�� | ľ̿ | C�� | ���ᱵ | D�� | �Ȼ��� |
 ��
��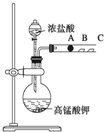 ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɣ���ͬѧ�������ͼװ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ����������
ij�о���ѧϰС�������һ��ʵ����̽��Ԫ�������ɣ���ͬѧ�������ͼװ������֤±��Ԫ�����ʵĵݱ���ɣ�A��B��C�����ֱ���մ��NaBr��Һ������ʪ��ĵ���KI��ֽ��ʪ���ֽ����֪������Ũ�������������ܷ�Ӧ����������