��Ŀ����
1����һ������������ͨ���ۺ�ˮ������Ӧ�����Եõ������������������������ֿ�����������Ӧ�����ɿ�����ϸ�����ۣ��������۾��кܸߵķ�Ӧ���ԣ��ڿ�������ײ��������ʱ��ȼ�գ������׳ơ�������������ֱ�����ͼ��ʾ�����������װ�ã���ȡ��������������͡�����������ʵ���б���ʹ����ͨ���ۺ�6Ħ/�����ᣬ�����Լ���ѡ��װ���б�Ҫ������̨�����С���Ȧ��ʯ�����������豸����ͼ�о�����ȥ����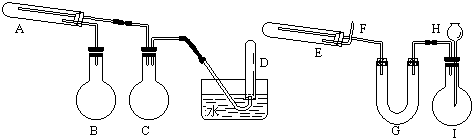
��д���пհף�
��1��ʵ�����ʱ�Թ�A��Ӧ������Լ�����ͨ���ۣ���ƿB����������ˮ��������ƿC������������ȫƿ�����Թ�D���ռ��õ�����������
��2��ʵ��ʱ��U��G��װ�м�ʯ�ң������������������е��Ȼ����ˮ��������ƿI�з����ķ�Ӧ�����ӷ���ʽΪ��Fe+2H+=Fe2++H2����
��3������װ���У���ʵ��ʱ��Ҫ���ȵ�������A��B��E�����������Ӧ����ĸ����
��4���Թ�E�з�����Ӧ�Ļ�ѧ����ʽ��Fe3O4+4H2$\frac{\underline{\;\;��\;\;}}{\;}$3Fe+4H2O��
���� һ������������ͨ���ۺ�ˮ������Ӧ�����Եõ����������������Ʊ�ʵ��װ�ÿ�֪��A��ΪFe�ۣ�������ƿB��ˮ���ṩˮ������C����ȫƿ��D����ˮ���ռ�������
�����������ֿ�����������Ӧ�����ɿ�����ϸ�����ۣ���ʵ��װ�ÿ�֪��E��ΪFe3O4��G�м�ʯ�Ҹ���������I�з���Fe������ķ�Ӧ��ȡ�������Դ������
��� �⣺��1��ʵ�����ʱ�Թ�A��Ӧ������Լ�����ͨ���ۣ���ƿB����������ˮ������A�з���3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2����ƿC������������ȫƿ�����Թ�D���ռ��õ�����������
�ʴ�Ϊ����ͨ���ۣ���ˮ����������ȫƿ��������
��2��ʵ��ʱ��U��G��װ�м�ʯ�ң������������������е��Ȼ����ˮ��������ƿI�з����ķ�Ӧ�����ӷ���ʽΪFe+2H+=Fe2++H2����
�ʴ�Ϊ�����������е��Ȼ����ˮ������Fe+2H+=Fe2++H2����
��3��A�з���3Fe+4H2O��g��$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2��B�����ṩˮ������E�з���Fe3O4+4H2$\frac{\underline{\;\;��\;\;}}{\;}$3Fe+4H2O��������װ���У���ʵ��ʱ��Ҫ���ȵ�������A��B��E��
�ʴ�Ϊ��A��B��E��
��4���Թ�E�з�����Ӧ�Ļ�ѧ����ʽ�ǣ��ʴ�Ϊ��Fe3O4+4H2$\frac{\underline{\;\;��\;\;}}{\;}$3Fe+4H2O��
���� ���⿼�����ʵ��Ʊ�ʵ�������ʵ�飬Ϊ��Ƶ���㣬����ʵ��װ�õ����á������ķ�Ӧ��ʵ�鼼��Ϊ���Ĺؼ������ط�����ʵ�������Ŀ��飬��Ŀ�ѶȲ���

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�| A�� | CH4+Cl2 $\stackrel{����}{��}$CH2Cl2+H2 | |
| B�� | 2CH3CH2OH+O2 $��_{��}^{����}$2CH3CHO+H2O | |
| C�� | CH3CH2OH+CH3COOH $\stackrel{Ũ����}{��}$CH3COOCH2CH3 | |
| D�� | H2C�TCH2+Br2��CH3CHBr2 |
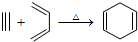 �����Ҫ�ϳ�
�����Ҫ�ϳ� ���õ�ԭʼԭ�Ͽ����ǣ�������
���õ�ԭʼԭ�Ͽ����ǣ�������| A�� | 2-��-1��3-����ϩ��2-��Ȳ | B�� | 1��3-���ϩ��2-��Ȳ | ||
| C�� | 2��3-����-1��3-���ϩ����Ȳ | D�� | 2��3-����-1��3-����ϩ��1-��Ȳ |

| A�� | ��ˮ��ʪ��pH��ֽ����ij��Һ��pH | |
| B�� | ����Ͳ��ȡ20 mL 0.5000 mol•L-1 H2SO4��Һ���ձ��У���ˮ80 mL�����Ƴ�0.1000 mol•L-1 H2SO4��Һ | |
| C�� | ʵ������ͼ����ʾװ����ȡ�������� | |
| D�� | ʵ������ͼ����ʾװ�ó�ȥCl2�е�����HCl |


 ��
��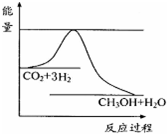 ������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ
������ʵ��д�����з�Ӧ���Ȼ�ѧ����ʽ