��Ŀ����
19��ijѧ������֪����y g�ı�����ȷ��ȡw g NaOH���壮����������ƽ�������Ϸţ�w+y��g���룬�����̵ı������м���NaOH���壬��ʱָ��ƫ���ұߣ���ͼ��ʾ����
��1���������IJ���Ӧ���Ǽ������������м����������ƹ��壬ʹָ��պ�ͣ���м�̶����ϣ�
��2������ȡ��w g NaOH�պÿ�����0.5mol•L-1NaOH��Һ500mL������������500mLNaOH��Һ����ʾ��ͼ���д�����ǣ��������ţ��٢ۢ�
��3����18mol•L-1Ũ��������100mL 3.0mol•L-1ϡ�����ʵ�鲽�����£�����Ũ����������16.7mL����ȡŨ�������õ���Ͳ�Ĺ����B����������ѡ��A��10mL��B��25mL��C��50mL��D��100mL����
��4����������������Ƶ�ϡ����Ũ���к�Ӱ�죿���á�ƫ��ƫС������Ӱ�족��д��
A������ƿ������ϴ�Ӻ������������ˮ��Ӱ��
B����ȡŨ�������Ͳ������ˮϴ�Ӻ����ձ�ƫ��
C����ȡŨ����ʱ���Ӷ���ƫС
D������ʱ���ӿ̶���ƫ��
���� ��1������ָ���ƫת�ж���������أ�ʵʩ��Ӧ�IJ���ʹָ��ָ�����룻
��2�����������׳��⣻ת����Һʱ�����������¶��ڿ̶������£��������ƫ��
��3������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬�ݴ˼�����ҪŨ��������������Ũ��������ѡ����Ͳ�Ĺ����Ͳ�����̱�Ũ��������Դɣ�
��4�������������������ʵ�������Һ�����Ӱ�죬����c=$\frac{n}{V}$�ж϶�������ҺŨ�ȵ�Ӱ�죻
��� �⣺��1��������ƽ�Ǹ��ݸܸ�ԭ���Ƴɵģ�ָ��ƫ����ߣ�˵�����̵������ߣ���ˣ���ָ��ƫ����ߣ�Ӧ���ұߵ���˿ñ�����ƶ���ָ������ƫת��˵������������������ʵ�����������ӦС�ļ���ҩƷ��ʹָ��ָ��̶����룬
�ʴ�Ϊ���������������м����������ƹ��壻ָ��պ�ͣ���м�̶����ϣ�
��2�����������׳��⣬Ӧ��С�ձ��г�����ת����Һʱ�����������¶��ڿ̶������£��������ƫ��Ũ��ƫС��
�ʴ�Ϊ���٢ۢޣ�
��3������ϡ�Ͷ��ɣ�ϡ��ǰ��������������ʵ������䣬����ҪŨ��������ΪV����V��18mol•L-1=100mL��3.0mol•L-1��ã�V=16.7mL
��Ҫ��ȡŨ����16.7mL����Ӧѡ��25mL����Ͳ��
�ʴ�Ϊ��16.7mL��B��
��4��A�������Ҫ���ݣ�����ƿ������ϴ�Ӻ������������ˮ������ҺŨ����Ӱ�죻
B����ȡŨ�������Ͳ������ˮϴ�Ӻ����ձ����������ʵ����ʵ���ƫ��������ҺŨ��ƫ��
C����ȡŨ����ʱ���Ӷ������������ʵ����ʵ���ƫС��������ҺŨ��ƫС��
D������ʱ���ӿ̶��ߣ�������Һ���ƫС��������ҺŨ��ƫ��
�ʴ�Ϊ��A����Ӱ�� B��ƫ��C��ƫС��D��ƫ��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�| A�� | 1��2 | B�� | 3��4 | C�� | 1��1 | D�� | 3��2 |
| A�� | H2O��g��=H2��g��+$\frac{1}{2}$O2��g����H=242 kJ?mol-1 | B�� | 2H2��g��+O2��g��=2H2O��g����H=-484 kJ?mol-1 | ||
| C�� | H2��g��+$\frac{1}{2}$O2��g��=H2O��1����H=-242 kJ?mol-1 | D�� | 2H2��g��+O2��g��=2H2O��g����H=+484 kJ?mol-1 |
| A�� |  | B�� |  | C�� |  | D�� |  |

 ��
�� ��
�� ��
��
 ��
�� ��
��
 ��
��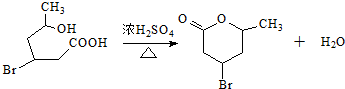 ��
�� ��2-��-1��3-����ϩ���������Ƣٵķ�Ӧ���õ��л���VI��VII���ṹ��ʽ�ֱ���ΪHCHO��CH3COCHO���������ʵ���֮����2��1��
��2-��-1��3-����ϩ���������Ƣٵķ�Ӧ���õ��л���VI��VII���ṹ��ʽ�ֱ���ΪHCHO��CH3COCHO���������ʵ���֮����2��1��