��Ŀ����
10���Ȼ����dz�����ˮ��������ij�Ȼ�����FeCl3•6H2O����Ʒ��������FeCl2���ʣ���Ҫ�ⶨ����FeCl3•6H2O������������ʵ�鰴���²�����У�
��֪�й����ӷ���ʽΪ��2Fe3++2I-��2Fe2++I2��I2+2S2O32-��2I-+S4O62-
��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ�̣�Һ����ֺ��ɫ����Ӧ�����ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+��
��2�����������õ��IJ����������ձ����������⣬�������С�100mL����ƿ����ͷ�ιܣ����������ƣ���
��3������������õ���������d��ѡ���ţ���
a��50mL�ձ� b��10mL��Ͳ �� c��20mL��Ͳ d��25mL�ζ���
ָʾ���ǵ�����Һ����ﵽ�ζ��յ�����������һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��4���ζ�ʱ������Ũ��Ϊ0.1000mol/L�ı�Na2S2O3��Һ18.17mL������Ʒ��FeCl3•6H2O����������Ϊ98.3%��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ�����õ��Լ���bd��ѡ���ţ���
a������ b����ˮ������c����ˮ d��˫��ˮ
��6������������²���ⶨ�Ȼ�����Ʒ����Ԫ�صĺ��������������գ�
�ٳ�����Ʒ �ڼ�ˮ�ܽ� �ۼ�������ˮ������ �ܹ��� ������ ���������к��ز�����
��ȱ�ٵ�һ��������ϴ�ӣ��ڹ���ǰ����Ҫ�����Ƿ������ȫ��������dz��������ڲ���Һ�У����백ˮ��Һ�۲����������ɣ�����������֤��������ȫ���ж��Ƿ���صı����������γ�����������Ȼ�������0.001g��
���� ��1���Ȼ���������Һ�����ˮ�м������������������壻
��2����������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
��3��������������Һ����ľ�ȷ�ȿ�֪��100.00mL����Һ��Ҫ����������ȡ���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ����Һ��ɫ��Ϊ��ɫ�Ұ���Ӳ���ɫ��
��4�����ݷ�Ӧ�Ķ�����ϵ����õ���ע����Һ����ı仯��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
��6������ʵ��������̷���������Ҫ���˺�ϴ�ӳ�ȥ��������ʣ������Ƿ������ȫ���������ϲ���Һ�м��백ˮ�۲��Ƿ��г������ɣ������������صı������γ���������ͬ��������0.001g��
��� �⣺��1��ȡ�����Ȼ�����Ʒ����50mL��ˮ�У�����Ƭ�̣�Һ����ֺ��ɫ�����ɵ��������������壬��Ӧ�����ӷ���ʽΪFe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
��2��������һ�����ʵ���Ũ�ȵ���Һ����Ҫ�������У���Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��
�ʴ�Ϊ��100mL����ƿ����ͷ�ιܣ�
��3��100.00mL����Һ��Ҫ����������ȡ���ձ��Ǵ�����ȡ����Ͳֻ�ܾ�ȷ��0.1mL�������õζ��ܾ�ȷ��0.01mL��ѡ�õζ�����ȡ��Һ100.00mL����Һ���������۱���ɫ�����Na2S2O3��Һ����͵ⵥ�ʷ�Ӧ�����һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ��d�� ���һ�α�Һ����ʱ����ƿ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
��4��2Fe3++2I-��2Fe2++I2��I2+2S2O32-��2I-+S4O62-��2FeCl3-6H2O��2Fe3+��I2��2S2O32-���ζ�ʱ��10.00ml��Һ�еⵥ������Ũ��Ϊ0.1000mol/L�ı�Na2S2O3��Һ18.17mL��FeCl3-6H2O�����ʵ���=0.1000mol/L��0.01817L=0.001817mol������Ʒ��100.00mL��Һ������FeCl3•6H2O�����ʵ���Ϊ0.01817mol����������=$\frac{0.01817mol��270.5g/mol}{5.0g}$��100%=98.3%��
�ʴ�Ϊ��98.3%��
��5��Ҫ����Ʒ�Ȼ����е�����FeCl2���ʳ�ȥ����Ҫ����������������������Ϊ�����ӣ�����������������������µ����ʣ�
a�����ۺ������ӷ�Ӧ�����ܺ� �������ӷ�Ӧ����a�����ϣ�
b����ˮ����������������Ϊ�����ӣ��Ҳ������µ����ʣ���b���ϣ�
c����ˮ�������������ӣ��������������ӣ���c�����ϣ�
d��˫��ˮ����������������Ϊ�����ӣ��������ⱻ��ԭΪˮ�����������ʣ���d���ϣ�
��ѡbd��
��6��ʵ��������̷���������Ҫ���˺�ϴ�ӳ�ȥ��������ʣ������Ƿ������ȫ���������ϲ���Һ�м��백ˮ�۲��Ƿ��г������ɣ������������صı����������γ���������ͬ��������0.001g��
�ʴ�Ϊ��ϴ�ӣ����������ڲ���Һ�У����백ˮ��Һ�۲����������ɣ�����������֤��������ȫ���������γ�����������Ȼ�������0.001g��
���� ���⿼����������ɺ����ʵ�ʵ����֤��ʵ��̽�������������仯�������ʵķ���Ӧ�ã����ʳ��ӣ��ζ�ʵ��ⶨ���ʺ����ļ���Ӧ���жϣ���Ŀ�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д� ��ͼΪԪ�����ڱ��ж����ڵ�һ���֣�����Ԫ�ؾ�Ϊ��ϡ������Ԫ�أ����й���������Ԫ�ؼ��仯�����˵������ȷ���ǣ�������
��ͼΪԪ�����ڱ��ж����ڵ�һ���֣�����Ԫ�ؾ�Ϊ��ϡ������Ԫ�أ����й���������Ԫ�ؼ��仯�����˵������ȷ���ǣ�������| A�� | ԭ�Ӱ뾶��Z��W��X��Y | |
| B�� | ��̬�⻯����ȶ��ԣ�Z��W��X��Y | |
| C�� | W����������ϼ��븺���ϼ۵ľ���ֵ��������� | |
| D�� | Z������������ˮ�������Ϊǿ�� |
| A�� | ��NO2ͨ��ˮ�У�����ɫ��ʧ��3NO2+H2O�T2HNO3+O2 | |
| B�� | ����Hg2+�ķ�ˮ�м���Na2S ����������Hg2++S2-�THg+S�� | |
| C�� | Na ��ˮ��Ӧ�������壺2Na+H2O�T2Na++2OH-+H2�� | |
| D�� | ��ȼú�м���ʯ��ʯ�ɼ��� SO2���ŷţ�2CaCO3+O2+2SO2$\frac{\underline{\;\;��\;\;}}{\;}$2CaSO4+2CO2 |

��1��װ��II���Թ��в�װ�κ��Լ����������Ƿ�ֹ��Һ������װ�â��У���ȫƿ����
��2��װ��II���Թܽ�����50���ˮԡ�У�Ŀ���Ƿ�ֹSO3Һ�������̣�
��3��װ��III��װ��IV��������̽����ʵ���������ɷ֣������ʵ����ƣ���д�����Լ���Ԥ����������ۣ�
| �����Լ� | Ԥ����������� |
| װ��III���Թ��м���BaCl2��Һ | ������ɫ������֤����������к���SO3�� |
| װ��IV���Թ��м������� KMnO4 ��Һ | ����Һ��ɫ��ȥ�� ֤����������к���SO2�� ����Һ��ɫ�����Ա仯�� ֤����������в���SO2 |
 �ڼס�������װ���У���ͷ�ι�������ij��Һ�壬��ƿ�г��루����룩��һ�����ʣ���ѹ��ͷ�ιܣ�����Һ�壬һ��ʱ�����װ���е������������ʹ��������Լ��ֱ���������ǣ�������
�ڼס�������װ���У���ͷ�ι�������ij��Һ�壬��ƿ�г��루����룩��һ�����ʣ���ѹ��ͷ�ιܣ�����Һ�壬һ��ʱ�����װ���е������������ʹ��������Լ��ֱ���������ǣ�������| A�� | �ף�Ũ�����ľ̿ �ң�Ũ��ˮ��SO2 | |
| B�� | �ף�˫��ˮ��MnO2 �ң�����ʳ��ˮ��HCl | |
| C�� | �ף����Ӻ�Na2CO3��Һ �ң�NaOH��Һ��Cl2 | |
| D�� | �ף�Ũ��������ǣ����м���ˮ�� �ң��Ȼ�������Һ������ |

| A�� | ��ϩ��H2��������ķ�Ӧ�����ȷ�Ӧ | |
| B�� | ����������ɼ�С��Ӧ����ЧӦ | |
| C�� | �����ܸı�ƽ��ת���ʣ����ܸı仯ѧ��Ӧ��ƽ�ⳣ�� | |
| D�� | ����������н����⻯���һ����ԭ�Ӻ�˫��̼ԭ���Ƚ�ϣ��õ��м��� |
| A�� |  ������ƿ��ת����Һ | B�� |  ������������ | ||
| C�� |  ̼���������ȷֽ� | D�� |  ��ȡ |
 �������һ�������Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ��ô������Խ�CO2��CH4ֱ��ת�������ᣬ��Ӧ����ʽΪ��CO2��g��+CH4��g��?CH3COOH��g����
�������һ�������Դ��Ҳ��һ����Ҫ�Ļ���ԭ�ϣ��ô������Խ�CO2��CH4ֱ��ת�������ᣬ��Ӧ����ʽΪ��CO2��g��+CH4��g��?CH3COOH��g����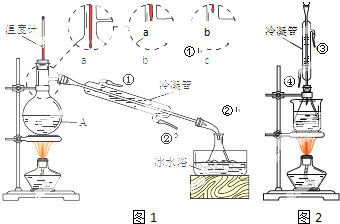 ������������в��ִ���ת��Ϊ���ᣬ�����γɸ���������ʹ�ƾ������������ζ�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30g/L���������������������ƣ�Ӧ������2.0g/L��
������������в��ִ���ת��Ϊ���ᣬ�����γɸ���������ʹ�ƾ������������ζ�����ұ��涨�����ʸ߶�Ũ���Ͱ���������������ƣ�Ӧ������0.30g/L���������������������ƣ�Ӧ������2.0g/L��