��Ŀ����
�����仯��������Ҫ��;����ۺ�������[Fe2��0H��n��S04��3-
]m��һ������Ч��ˮ��������������������أ��������Ļ��ϼ�Ϊ+6����һ����Ҫ��ɱ����������ij����С���� �����·����Ʊ��������ֲ�Ʒ��
��ش��������⣺
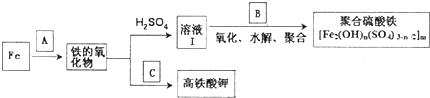
��1����AΪH20��g�������Եõ�Fe304��д��Fe��H20��g����Ӧ�Ļ�ѧ����ʽ�ĵ���ʽ�� ��
��2����BΪNaCl03��ϡ���ᣬд��������Fe2+�����ӷ���ʽ����ԭ����ΪCD���� ��
��3����CΪKKO3��KOH�Ļ���д������Fe2O3���ȹ����Ƶø�����⛵Ļ�ѧ����ʽ����ƽ��
Fe2O3+ KNO3+ KOH- + KNO2+
��4��Ϊ�ⶨ��ҺI����Ԫ�ص��ܺ�����ʵ�������ȷ��ȡ20.00mL��ҺI�ڴ�����ƿ�У���������H202������pH��3�����ȳ�ȥ����H202���������KI��ַ�Ӧ������O.1OOOmol?L-1 Na2S2O3����Һ�ζ����յ㣬���ı���Һ20.00mL��
��֪��2Fe3++2I-�T2Fe2++I2 I2+2S2O32-�T2I-+S4O62-
��д���ζ�ѡ�õ�ָʾ�� ���ζ��յ�۲쵽������ ��
����Һ������Ԫ�ص��ܺ���Ϊ g?L-1�����ζ�ǰ��Һ��H202û�г��������ⶨ����Ԫ�صĺ������� ���ƫ�ߡ���ƫ�͡������䡱����
��5�����ʵ�鷽����������ҺI�е�Fe2+��Fe3+ ��
| n |
| 2 |

��ش��������⣺
��1����AΪH20��g�������Եõ�Fe304��д��Fe��H20��g����Ӧ�Ļ�ѧ����ʽ�ĵ���ʽ��
��2����BΪNaCl03��ϡ���ᣬд��������Fe2+�����ӷ���ʽ����ԭ����ΪCD����
��3����CΪKKO3��KOH�Ļ���д������Fe2O3���ȹ����Ƶø�����⛵Ļ�ѧ����ʽ����ƽ��
��4��Ϊ�ⶨ��ҺI����Ԫ�ص��ܺ�����ʵ�������ȷ��ȡ20.00mL��ҺI�ڴ�����ƿ�У���������H202������pH��3�����ȳ�ȥ����H202���������KI��ַ�Ӧ������O.1OOOmol?L-1 Na2S2O3����Һ�ζ����յ㣬���ı���Һ20.00mL��
��֪��2Fe3++2I-�T2Fe2++I2 I2+2S2O32-�T2I-+S4O62-
��д���ζ�ѡ�õ�ָʾ��
����Һ������Ԫ�ص��ܺ���Ϊ
��5�����ʵ�鷽����������ҺI�е�Fe2+��Fe3+
���㣺�Ʊ�ʵ�鷽�������,������ԭ��Ӧ����ʽ����ƽ
ר�⣺ʵ�������
��������1������ˮ�����ڸ����·�Ӧ����������������������
��2����Һ���к����������ӣ����������£�ClO3-����Fe2+ΪFe3+����������ԭΪCl-�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ��
��3����CΪKKO3��KOH�Ļ�����Fe2O3���ȹ����Ƶø�����أ���ȱ������ΪK2FeO4��H2O�����ݵ�ʧ������ȡ������غ���ƽ��
��4���ٷ�Ӧԭ�����еⵥ�����ɺ����ģ��ⵥ������������ʾ��ɫ����ѡ����Ϊָʾ�������ⵥ����ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ���ݴ��жϵζ��յ㣻
�ڸ��ݷ�Ӧ2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-�ҳ���ϵʽFe3+��S2O32-��Ȼ����ݹ�ϵʽ����������ӵ����ʵ������ٸ���c=
�������Ԫ�غ���������˫��ˮ������S2O32-�����ʵ�����Ӱ���ж���
��5��������Һ�е�Fe3+����KSCN��Һ��������Һ�е�Fe2+�������Ը��������Һ��K3[Fe��CN��]6��Һ��
��2����Һ���к����������ӣ����������£�ClO3-����Fe2+ΪFe3+����������ԭΪCl-�����ݵ�ʧ������ȡ�����غ㡢�����غ���ƽ��
��3����CΪKKO3��KOH�Ļ�����Fe2O3���ȹ����Ƶø�����أ���ȱ������ΪK2FeO4��H2O�����ݵ�ʧ������ȡ������غ���ƽ��
��4���ٷ�Ӧԭ�����еⵥ�����ɺ����ģ��ⵥ������������ʾ��ɫ����ѡ����Ϊָʾ�������ⵥ����ȫ��Ӧ����Һ����ɫ��Ϊ��ɫ���ݴ��жϵζ��յ㣻
�ڸ��ݷ�Ӧ2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-�ҳ���ϵʽFe3+��S2O32-��Ȼ����ݹ�ϵʽ����������ӵ����ʵ������ٸ���c=
| n |
| V |
��5��������Һ�е�Fe3+����KSCN��Һ��������Һ�е�Fe2+�������Ը��������Һ��K3[Fe��CN��]6��Һ��
���
�⣺��1��Fe��ˮ�����ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O��g��
Fe304+4H2��
�ʴ�Ϊ��3Fe+4H2O��g��
Fe304+4H2��
��2�������������̿�֪�����������������ᷴӦ��������������������������Һ���к����������ӣ����������£�ClO3-����Fe2+ΪFe3+����������ԭΪCl-�����ݻ��ϼ����������ƽ����ƽ������ӷ���ʽΪ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O��
�ʴ�Ϊ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O��
��3��KNO3��KOH�Ļ���д������Fe2O3���ȹ����Ƶ�K2FeO4����ȱ������һ��ΪK2FeO4��K2FeO4����Ԫ�ػ��ϼ�Ϊ+6�����������Ӵ�+3�۱�Ϊ+6�ۣ����ϼ�����3�ۣ����ϼ���������3��2=6�ۣ�KNO3��NԪ�ش�+5��ΪKNO2�е�+3�ۣ����ϼ۽���2�ۣ��ϼ���С������Ϊ6��������������ϵ��Ϊ1��KNO3��ϵ��Ϊ3��Ȼ����������غ㶨����ƽ����ƽ��ķ���ʽΪ��Fe2O3+3KNO3+2KOH=K2FeO4+3KNO2+H2O��
�ʴ�Ϊ��1��3��4��2��K2FeO4��3��2��H2O��
��4����Fe3+����I-����I2���������������ѡ�������Һ��ָʾ�������������һ�������������Һʱ����ɫ��ʧ���Ұ���Ӳ���ɫ˵�����յ㣬
�ʴ�Ϊ�����ۣ���Һ����ɫ����ɫ�ұ��ְ���Ӳ���ɫ��
����2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-�ɵã�Fe3+��S2O32-����n��Fe3+��=n��S2O32-��=0.1000mol/L��0.02L=0.002mol����Ԫ���ܺ���Ϊ��
=5.6g/L��
H2O2Ҳ������I-����I2����������������û�г����������������������Һ���ƫ��������ƫ�ߣ�
�ʴ�Ϊ��5.6��ƫ�ߣ�
��5��������ҺI�е�Fe2+��Fe3+�ķ���Ϊ��ȡ��Һ���������Թ��У����������ữ���ټ�����������Һ����Һ��ɫ������ɫ��ɻ�ɫ���dz������Fe2+����ȡ��Һ���������Թ��У��������軯����Һ������ɫ�������ɣ�����Fe2+������ȡ��Һ���������Թ��У��μ����軯����Һ�������Ϊ��ɫ��֤������������
�ʴ�Ϊ��ȡ��Һ���������Թ��У����������ữ���ټ�����������Һ����Һ��ɫ������ɫ��ɻ�ɫ���dz������Fe2+����ȡ��Һ���������Թ��У��������軯����Һ������ɫ�������ɣ�����Fe2+������ȡ��Һ���������Թ��У��μ����軯����Һ�������Ϊ��ɫ��֤�����������ӣ�
| ||
�ʴ�Ϊ��3Fe+4H2O��g��
| ||
��2�������������̿�֪�����������������ᷴӦ��������������������������Һ���к����������ӣ����������£�ClO3-����Fe2+ΪFe3+����������ԭΪCl-�����ݻ��ϼ����������ƽ����ƽ������ӷ���ʽΪ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O��
�ʴ�Ϊ��6Fe2++ClO3-+6H+�T6Fe3++Cl-+3H2O��
��3��KNO3��KOH�Ļ���д������Fe2O3���ȹ����Ƶ�K2FeO4����ȱ������һ��ΪK2FeO4��K2FeO4����Ԫ�ػ��ϼ�Ϊ+6�����������Ӵ�+3�۱�Ϊ+6�ۣ����ϼ�����3�ۣ����ϼ���������3��2=6�ۣ�KNO3��NԪ�ش�+5��ΪKNO2�е�+3�ۣ����ϼ۽���2�ۣ��ϼ���С������Ϊ6��������������ϵ��Ϊ1��KNO3��ϵ��Ϊ3��Ȼ����������غ㶨����ƽ����ƽ��ķ���ʽΪ��Fe2O3+3KNO3+2KOH=K2FeO4+3KNO2+H2O��
�ʴ�Ϊ��1��3��4��2��K2FeO4��3��2��H2O��
��4����Fe3+����I-����I2���������������ѡ�������Һ��ָʾ�������������һ�������������Һʱ����ɫ��ʧ���Ұ���Ӳ���ɫ˵�����յ㣬
�ʴ�Ϊ�����ۣ���Һ����ɫ����ɫ�ұ��ְ���Ӳ���ɫ��
����2Fe3++2I-�T2Fe2++I2��I2+2S2O32-�T2I-+S4O62-�ɵã�Fe3+��S2O32-����n��Fe3+��=n��S2O32-��=0.1000mol/L��0.02L=0.002mol����Ԫ���ܺ���Ϊ��
| 56g/mol��0.002mol |
| 0.02L |
H2O2Ҳ������I-����I2����������������û�г����������������������Һ���ƫ��������ƫ�ߣ�
�ʴ�Ϊ��5.6��ƫ�ߣ�
��5��������ҺI�е�Fe2+��Fe3+�ķ���Ϊ��ȡ��Һ���������Թ��У����������ữ���ټ�����������Һ����Һ��ɫ������ɫ��ɻ�ɫ���dz������Fe2+����ȡ��Һ���������Թ��У��������軯����Һ������ɫ�������ɣ�����Fe2+������ȡ��Һ���������Թ��У��μ����軯����Һ�������Ϊ��ɫ��֤������������
�ʴ�Ϊ��ȡ��Һ���������Թ��У����������ữ���ټ�����������Һ����Һ��ɫ������ɫ��ɻ�ɫ���dz������Fe2+����ȡ��Һ���������Թ��У��������軯����Һ������ɫ�������ɣ�����Fe2+������ȡ��Һ���������Թ��У��μ����軯����Һ�������Ϊ��ɫ��֤�����������ӣ�
���������⿼�������ʵ��Ʊ���������ơ��������ӵļ��顢������ԭ��Ӧ��ƽ����ѧ�����֪ʶ����Ŀ�Ѷ��еȣ�����֪ʶ��϶ࡢ�ۺ��Խ�ǿ����һ��������ϵ���Ŀ����ֿ�����ѧ���ķ������������������Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
 ������ϵ�д�
������ϵ�д�
�����Ŀ
 ij��ɫ���������O2��CO��CO2��HCl��NH3��NO��Br2�е�һ�ֻ�����ɣ�ͨ��ͼ��ϴ��ƿ������������٣���װ��ǰ�������ֱ����������ͨ������ܣ���ʢ��Na2O2��������ʺ���ɫ���������ƶ���ȷ���ǣ�������
ij��ɫ���������O2��CO��CO2��HCl��NH3��NO��Br2�е�һ�ֻ�����ɣ�ͨ��ͼ��ϴ��ƿ������������٣���װ��ǰ�������ֱ����������ͨ������ܣ���ʢ��Na2O2��������ʺ���ɫ���������ƶ���ȷ���ǣ�������| A��ԭ������һ����NO��O2 |
| B��ԭ������һ����NH3��NO��CO2��CO |
| C��ԭ������һ��û��CO |
| D��ԭ������һ��û��HCl��Br2��O2 |
 ��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ�������
��ͼ��ʾ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ�ιܽ�ͷ��ʹҺ��Y����ƿ�У���һ����ɼ�С����a��������X��Һ��Y�������ǣ�������| X | Y | |
| A | HCl | ����ʳ��ˮ |
| B | CO2 | ϡH2SO4 |
| C | SO2 | NaOH |
| D | H2S | ��ˮ |
| A��A | B��B | C��C | D��D |
����˵����ȷ���ǣ�������
| A��ֻ��һ��Ԫ����ɵ����ʣ�һ���Ǵ����� |
| B������������һ�����Ƿǽ��������� |
| C���ǽ���Ԫ����ɵĻ�����һ�����й��ۼ� |
| D�����������о��ֱ�ֻ����һ��Ԫ�أ���������������������ɵ�����һ���Ǵ����� |
���й�ϵʽ������ǣ�������
| A����Ũ�ȵ�HCN��ҺNaCN��Һ�������ϣ�������ҺpH��7������Һ������Ũ�ȣ�c��Na+ ����c��CN-����c��OH- ����c��H+ �� | ||||
| B��0.4mol?L-1ijһԪ��HA��Һ��0.2 mol?L-1NaOH��Һ�������ϵ���Һ�У�2c��OH- ��+c��A- ��=2c��H+ ��+c��HA�� | ||||
| C�������µ�Ũ�ȵ�Na2SO3��NaHSO3��Һ�������Ϻ���Һ��pH=7.2��������������Һ������ʱ��c��Na+ ����c��HSO3-����c��SO32-����c��H+ ���Tc��OH- �� | ||||
D����������HX��HY��Ϻ���Һ�е�c��H+ ��Ϊ��KaΪ����ƽ�ⳣ������c��H+ ��=
|
 ijͬѧ�������ͼװ�ã��г�����ʡ�ԣ�����ϵ��ʵ��ʱ����ҩƷA��μ��뵽����B�У����������ʵ��ش����⣮
ijͬѧ�������ͼװ�ã��г�����ʡ�ԣ�����ϵ��ʵ��ʱ����ҩƷA��μ��뵽����B�У����������ʵ��ش����⣮ ���а�ˮ�����ᡢ���ᡢ�Ȼ��������Һ����ش��������⣺
���а�ˮ�����ᡢ���ᡢ�Ȼ��������Һ����ش��������⣺ ��˫ϩ�ϳɣ�Ҳ�ɱ�ʾΪ
��˫ϩ�ϳɣ�Ҳ�ɱ�ʾΪ ��
��


