��Ŀ����
6�� ��θ�ᣨ��Ҫ�ɷ������ᣩ���࣬������ܶ�θ������ͼΪij����ҩ��װ��ǩ�ϵIJ������֣���ش��������⣮
��θ�ᣨ��Ҫ�ɷ������ᣩ���࣬������ܶ�θ������ͼΪij����ҩ��װ��ǩ�ϵIJ������֣���ش��������⣮��1����ҩ��������̷�����ԭ����ҩ���θ���ֽӴ���Ѹ�ٷ���ҩЧ��
��2��ijͬѧΪ�ⶨ��ҩ���������������������������¼���������ȡһƬҩƬ��ҩƬ����Ϊ0.5g����������20mL����ˮ��Ȼ������������Ϊ5%���ܶ�Ϊ1.02g/mL��������з�Ӧ�����������ɷֲ������ᷴӦ�������ʵ���з�Ӧ�����������Ϊ6.0mL��ͨ�����㣺
���жϸ�ҩƬ�����������ĺ����Ƿ����ǩ�����
��ȷ����ҩƬ�������������������������٣�
���� ��1������ȷ����⣬�����������ϵͳ���ص��Ӱ�컯ѧ��Ӧ���ʵ����ؿ��ǣ�
��2��θ�����Ҫ�ɷ������ᣬ���Ժ�Al��OH��3��Ӧ��д����Ӧ����ʽ���ɣ����ݻ�ѧ��Ӧ����ʽ�ó�������֮��������ȣ��г�����ʽ���������Al��OH��3�������������ǩ�ϵĺ����Ƚϣ��Ϳ��жϸ�ҩƬ�����������ĺ����Ƿ�ﵽ��ע��Ȼ���������������ʽ�Ϳɼ������ҩƬ����������������������
��� �⣺��1������һ��������ı����������飩��������Ӧ���ʣ�������̼��þƬ��С����״������������θ��ĽӴ��棬������ڱ������������ҩЧ��
�ʴ�Ϊ��ҩ���θ���ֽӴ���Ѹ�ٷ���ҩЧ��
��2����θ�����Ҫ�ɷ������ᣬ���Ժ�Al��OH��3�����кͷ�Ӧ������������������Ϊx���뷴Ӧ��HCl������Ϊ��1.02g/mL��6.0mL��5%��0.306g
Al��OH��3+3HCl=AlCl3+3H2O
78 109.5
x 0.306g
��֮�ã�x��0.218g
0.218g=218mg��250mg
�ʸ�ҩƬ�����������ĺ���û�дﵽ��ע��
�𣺸�ҩƬ�����������ĺ���û�дﵽ��ע��
�ڸ�ҩƬ��������������������Ϊ $\frac{0.218g}{0.5g}$��100%��43.6%��
�𣺸�ҩƬ����������������������43.6%��
���� ������Ҫ����ѧ���������кͷ�Ӧ��Ӱ�컯ѧ��Ӧ���ʵ����ص�֪ʶ�������������Լ����û�ѧ����ʽ������������ʽ���м����������ѧ�������������֪��������ȷ��д��ѧ����ʽ��������ȷ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���1��������Ӧ�Ļ�ѧ����ʽΪ2H2SO4��Ũ��+C$\frac{\underline{\;\;��\;\;}}{\;}$2SO2��+CO2��+2H2O��
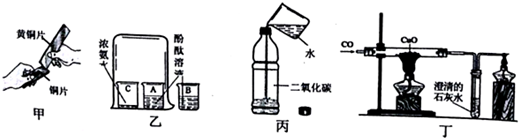
��2��������ͼ���и�װ�����һ��ʵ�飬����֤������Ӧ�������ĸ��ֲ��
| ��� | �� | �� | �� | �� |
| װ�� |  |  |  |  |
g��c��d��a��b��f��
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ�е���Һ��ɫ��Cƿ�е���Һ����ɫ��Aƿ�е���Һ�������Ǽ���SO2���壻Bƿ�е���Һ������������SO2���壻Cƿ�е���Һ�������Ǽ���SO2�����Ƿ���ɾ���
��4��װ�â������ӹ���ҩƷ����ˮ����ͭ������֤�IJ�����H2O��
��5��װ�â�����ʢ��Һ�dz���ʯ��ˮ������֤�IJ�����CO2��
| A�� | �����ԣ�ClO-��I2��SO42- | |
| B�� | ��ɫ��ʧ��ԭ����Na2SO3��Һ��Ӧ����SO2����Ư���� | |
| C�� | ����KI��Һ��������ΪI-��ClO-����ΪI2��I2�����۱��� | |
| D�� | ����Na2SO3��Һ������ˮ����ˮ��ɫ |
| A�� | ��ʵ���л�ͭƬ����ͭƬ�Ͽ̻����ۼ�����˵����ͭ��Ӳ�ȱ�ͭƬ�� | |
| B�� | ��ʵ��ȿ���˵�������ڲ�ͣ���˶��ţ��ֿ���˵����ˮ�Լ��� | |
| C�� | ��ʵ��ȿ���˵��������̼������ˮ���ֿ���˵��������̼�������� | |
| D�� | ��ʵ��ȿ���˵��һ����̼���л�ԭ�ԣ��ֿ���˵��һ����̼���п�ȼ�� |
| A�� | ԭ�Ӽ������ӵĺ�����Ӳ������ڸ�Ԫ�����ڵ������� | |
| B�� | Ԫ�����ڱ��д�IIIB�嵽IIB�� 10�����е�Ԫ�ض��ǽ���Ԫ�� | |
| C�� | �������ϡ������ԭ�ӵ���������������8 | |
| D�� | ͬһԪ�صĸ���ͬλ�ص��������ʡ���ѧ���ʾ���ͬ |
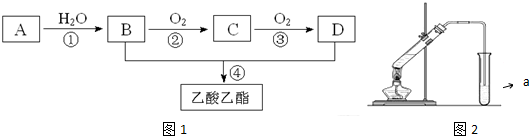
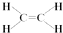 ��C�Ľṹ��ʽ��CH3CHO��
��C�Ľṹ��ʽ��CH3CHO��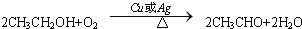 ����Ӧ���ͣ�������Ӧ��
����Ӧ���ͣ�������Ӧ��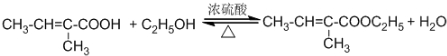 ��Ϊ�Ӹ�ʵ���Ļ�������з���������������Ҳ��Թ�����ѡ�õ��Լ�a�DZ���̼������Һ��a�Լ����������к����ᡢ�����Ҵ�������������������Һ�е��ܽ�ȣ������ڷֲ�������
��Ϊ�Ӹ�ʵ���Ļ�������з���������������Ҳ��Թ�����ѡ�õ��Լ�a�DZ���̼������Һ��a�Լ����������к����ᡢ�����Ҵ�������������������Һ�е��ܽ�ȣ������ڷֲ�������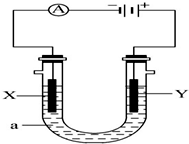 ���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����ش��������⣺
���ԭ���ڻ�ѧ��ҵ���й㷺Ӧ�ã���ͼ��ʾһ�����أ�X��Y���Ƕ��Ե缫��a�DZ���NaCl��Һ��ʵ�鿪ʼʱ��ͬʱ�����߸����뼸�η�̪��Һ����ش��������⣺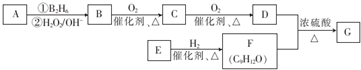
 ���÷�Ӧ�ķ�Ӧ����Ϊȡ����Ӧ��������Ӧ
���÷�Ӧ�ķ�Ӧ����Ϊȡ����Ӧ��������Ӧ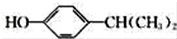 ��
��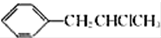 Ϊԭ��Ҳ�ɺϳ�F����ο���Ŀ�е������Ϣд����Ӧ�ĺϳ�·��ͼ����Ӧ�����е��Լ�д�ڼ�ͷ�Ϸ�������д�ڼ�ͷ�·�����
Ϊԭ��Ҳ�ɺϳ�F����ο���Ŀ�е������Ϣд����Ӧ�ĺϳ�·��ͼ����Ӧ�����е��Լ�д�ڼ�ͷ�Ϸ�������д�ڼ�ͷ�·�����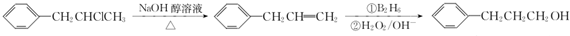 ��
��