��Ŀ����
19���������л�ѧ��������Ʒ���ٲ����� ���ձ� ��������ƽ ����Ͳ ��ҩ�� ��ͷ�ι� ������ƿ ���ǩֽ ��ϸ���Լ�ƿ��1������������Ҫ����460mL 0.1moL/L NaOH��Һ����Ӧ��ȡ����NaOH2.0g��
��2�������ϳ������NaOH����������Һ�����ã���������Һ�Ĺ���˳������������������Ʒ��ѡȡ��Ҫ���������ظ��IJ��ƣ������������Ǣڢܢ٢ߢޢ�ࣻ
��3�����ƹ����У�Ӧ������NaOH�ܽ����ȴ�����µ���Һת�Ƶ�500ml����ƿ�У���Һ��ӽ�����ƿ�̶���1-2cm�������ý�ͷ�ιܶ��ݣ�
��4����ʵ��������������������ܵ�����ҺŨ��ƫ�ߵ���A��E������ĸ����
A���������������ܽ�ʱδ��ȴ�����£��Ϳ�ʼת�ơ�ϴ���Լ����ݣ�
B��ת��ʱ��С����������Һ����������ƿ�⣻
C������ʱ��������ƿ�̶��ߣ�
D������ʱ��ˮ�����˿̶��ߣ������ý�ͷ�ι���ȥ�����ˮ��
E�����ƺú��ֳ���ʱ���õ�������ƽ�����������ˣ�
���� ��1������������m=CVM�����㣻
��2�����������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ��������
��3��������ƿ�������ȣ�����Һʱ�������������������������ݶ��ݵIJ�����������
��4��������c=n/V��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1����û��460mL����ƿ��ѡ��500mL���������������Ƶ�����m=CVM=0.1mol/L��0.5L��40g/mol=2.0g���ʴ�Ϊ��2.0��
��2�����������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪�����������˳��Ϊ�ڢܢ٢ߢޢ�࣬
�ʴ�Ϊ���ڢܢ٢ߢޢ�ࣻ
��3��������ƿ�������ȣ���������������ˮ���ȣ���Ҫ��������ȴ������Ȼ��ת����500mL����ƿ�У�����Һʱ������������������������ֹ��Һ����������ʱ��Ҫ��������ƿ�е�ˮ����Һ����̶���1-2cmʱ�����ý�ͷ�ι���μ��룬����Һ����̶������У�
�ʴ�Ϊ��500ml����ƿ�� ������ 1-2cm�� ��ͷ�ιܣ�
��4����A���������������ܽ�ʱδ��ȴ�����£��Ϳ�ʼת�ơ�ϴ���Լ����ݣ�����ȴ����Һ���ƫС��Ũ��ƫ�ߣ���A��ȷ��
B��ת��ʱ��С����������Һ����������ƿ�⣬��ᵼ�����ʵ���ʧ��Ũ��ƫС����B����
C������ʱ��������ƿ�̶��ߣ�����Һ���ƫ��Ũ��ƫ�ͣ���C����
D������ʱ��ˮ�����˿̶��ߣ������ý�ͷ�ι���ȥ�����ˮ���������IJ�ֹ��ˮ���������ʣ���Ũ��ƫ�ͣ���D����
E�����ƺú��ֳ���ʱ���õ�������ƽ�����������ˣ�������������������������ʵ���������Ũ��ƫ�ߣ���E��ȷ��
��ѡAE��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���

| A�� | ���ܺ���Ag+��Al3+��NH${\;}_{4}^{+}$ | |
| B�� | һ������Cl-�����ܺ���NO${\;}_{3}^{-}$ | |
| C�� | һ������AlO${\;}_{2}^{-}$��NH${\;}_{4}^{+}$��CO${\;}_{3}^{2-}$ | |
| D�� | ���ܺ���Fe3+��Fe2+��һ������AlO${\;}_{2}^{-}$��CO${\;}_{3}^{2-}$ |
| A�� | ת����Һ��δϴ���ձ��Ͳ�������ֱ�Ӷ��� | |
| B�� | NaOH�����ܽ������ת������ƿ | |
| C�� | ������ƿ�н��ж���ʱ���ӿ̶��� | |
| D�� | ���ݺ������ƿ��תҡ�ȣ�����Һ����ڿ̶ȣ��ٲ��伸��ˮ���̶� |
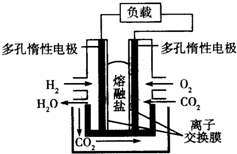
| A�� | CO2�ڵ�ط�Ӧ�����в����뷴Ӧ | |
| B�� | ����ܷ�ӦΪ2H2O�TO2��+2H2�� | |
| C�� | �����ӽ���ĤΪ�����ӽ���Ĥ | |
| D�� | ���������ӦʽΪO2+2CO2+4e-�T2CO32- |
| A�� | ��̬ˮҺ�� | B�� | �ƾ�ȼ�� | ||
| C�� | ̼��Ƹ��·ֽ� | D�� | �����������Ȼ�立�Ӧ |
 PM2.5�����������ж����к����ʣ����������ι⻯ѧ�������⻯ѧ�����к���NOx��HCOOH��
PM2.5�����������ж����к����ʣ����������ι⻯ѧ�������⻯ѧ�����к���NOx��HCOOH��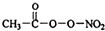 ��PAN���ȶ�����Ⱦ�
��PAN���ȶ�����Ⱦ�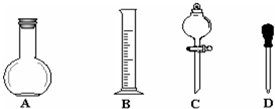 ʵ��������1000mL 0.1mol•L-1��Na2CO3��Һ����ش��������⣺
ʵ��������1000mL 0.1mol•L-1��Na2CO3��Һ����ش��������⣺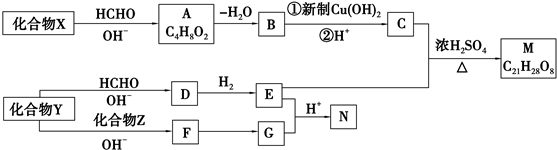
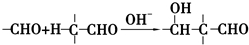
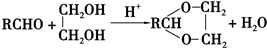
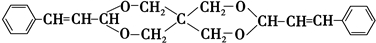
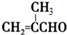 +2Cu��OH��2+NaOH$\stackrel{��}{��}$
+2Cu��OH��2+NaOH$\stackrel{��}{��}$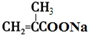 +Cu2O��+3H2O��
+Cu2O��+3H2O��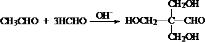 ��
��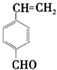 ��
�� ��
��