��Ŀ����
�������л�ѧ�������ļ��㣬�����ֱ������ո���
��1��0.2molij���ʵ�����Ϊ12.8g�������ʵ�Ħ������Ϊ
��2����������Ϊ36.5%�����ᣨ�ܶ�Ϊ1.19g?cm-3��������Һ�����ʵ���Ũ��Ϊ mol/L��
��3����״����5.6L NH3������ԼΪ g������5.6L NH3����100mLˮ�У����ð�ˮ���ܶ�Ϊ��g?cm-3����ð�ˮ�а������ʵ���Ũ�ȵļ���ʽ�� mol/L��ֻҪ���г�����ʽ����
��1��0.2molij���ʵ�����Ϊ12.8g�������ʵ�Ħ������Ϊ
��2����������Ϊ36.5%�����ᣨ�ܶ�Ϊ1.19g?cm-3��������Һ�����ʵ���Ũ��Ϊ
��3����״����5.6L NH3������ԼΪ
���㣺���ʵ�������ؼ���,���ʵ���Ũ�ȵ���ؼ���
ר�⣺������
��������1������M=
���㣻
��2������c=
���㣻
��3������n=
=
����������������Һ�����������V=
��c=
���㣮
| m |
| n |
��2������c=
| 1000�Ѧ� |
| M |
��3������n=
| V |
| Vm |
| m |
| M |
| m |
| �� |
| n |
| V |
���
�⣺��1��M=
=
=64g/mol���ʴ�Ϊ��64g/mol��
��2��c=
=
=11.9mol/L���ʴ�Ϊ��11.9��
��3��n��NH3��=
=0.25mol��m��NH3��=0.25mol��17g/mol=4.25g��
����5.6L NH3����100mLˮ�У�m����Һ��=100g+4.25g��
V����Һ��=
mL��
c=
=
mol/L
�ʴ�Ϊ��4.25��
��
| m |
| n |
| 12.8g |
| 0.2mol |
��2��c=
| 1000�Ѧ� |
| M |
| 1000��1.19g/L��36.5% |
| 36.5g/mol |
��3��n��NH3��=
| 5.6L |
| 22.4L/mol |
����5.6L NH3����100mLˮ�У�m����Һ��=100g+4.25g��
V����Һ��=
| 100+4.25 |
| �� |
c=
| 0.25mol | ||
|
| 250�� |
| 100+4.25 |
�ʴ�Ϊ��4.25��
| 250�� |
| 100+4.25 |
���������⿼�����ʵ�������ؼ��㣬������ѧ���ķ��������ͼ��������Ŀ��飬Ϊ��Ƶ���㣬ע����ؼ��㹫ʽ�����ã��ѶȲ���

��ϰ��ϵ�д�
 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�
�����Ŀ
 pH=11��x��y���ּ���Һ��5mL���ֱ�ϡ����500mL����pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
pH=11��x��y���ּ���Һ��5mL���ֱ�ϡ����500mL����pH����Һ�����V���Ĺ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A����x��y���������a��ֵһ��С��9 |
| B��ϡ�ͺ�x��Һ��ˮ�ĵ���̶ȱ�y��Һ��ˮ����̶�С |
| C����ȫ�к�x��y����Һʱ������ͬŨ��ϡ��������V��x����V��y�� |
| D����x��y��һԪ�������ʵ���Ũ�ȵ�y����������Һ��pH��x����������ҺС |
�����£����и���Һ����������ȷ���ǣ�������
| A��0.1mol?L-1�Ĵ�������Һ20 mL��0.1mol?L-1����10 mL��Ϻ���Һ������c ��CH3COO-����c ��Cl-����c ��CH3COOH����c ��H+�� | ||||
B��pH=7��NaHSO3��Na2SO3�����Һ�У�3c��Na+��=c��HSO
| ||||
| C����֪����HF��CH3COOH��pH��ȵ�NaF��CH3COOK��Һ�У�[c��Na+��-c��Fһ��]��[c��K+��-c��CH3COO-��] | ||||
| D����֪ij�¶���Ksp��CH3COOAg��=2.8��10-3��Ũ�Ⱦ�Ϊ0.1 mol?L-1��AgNO3��Һ��CH3COONa��Һ��������һ���ܲ���CH3COOAg���� |
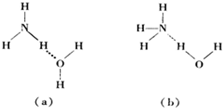 ��Ҫ�����
��Ҫ�����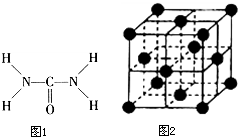 ��ͼ1����֪���صĽṹʽΪ�����ؿ��������л����ʣ���Ҫ�������������ᣬ�����غ���������ѧʽΪ[Fe��H2NCONH2��6]
��ͼ1����֪���صĽṹʽΪ�����ؿ��������л����ʣ���Ҫ�������������ᣬ�����غ���������ѧʽΪ[Fe��H2NCONH2��6]


