��Ŀ����
�������ֳƻ����������������ԭ�ϣ�����Ҫ�ɷ�ΪFeS2��850�桫900��ʱ�������������������գ����ܷ������з�Ӧ���������N2��O2�����Ϊ4��1����
3FeS2+8O2��Fe3O4+6SO2 ��
4FeS2+11O2��2Fe2O3+8SO2 ��
��1������32%����������Ʒ�����ʲ�������FeS2�ĺ���Ϊ ��
��2����120kg������FeS2����ʽ��ȫ��Ӧ��������״����SO2 m3��
3FeS2+8O2��Fe3O4+6SO2 ��
4FeS2+11O2��2Fe2O3+8SO2 ��
��1������32%����������Ʒ�����ʲ�������FeS2�ĺ���Ϊ
��2����120kg������FeS2����ʽ��ȫ��Ӧ��������״����SO2
���㣺��ѧ����ʽ���йؼ���
ר�⣺������
��������1��FeS2������������FeS2��SԪ����������=��������Ʒ��SԪ������������
��2�����ݷ���ʽ�������ɶ���������������ٸ���n=
���������������ʵ���������V=nVm�����������
��2�����ݷ���ʽ�������ɶ���������������ٸ���n=
| m |
| M |
���
�⣺��1��FeS2������������FeS2��SԪ����������=��������Ʒ��SԪ��������������FeS2����������=
=60%���ʴ�Ϊ��60%��
��2�������ɶ������������Ϊm����
4FeS2+11O2=2Fe2O3+8SO2
4��120 8��64
120kg m
m=
=128kg
�ʶ�����������ʵ���=
=2000mol������¶�����������=2000mol��22.4L/mol=44.8��1000L=44.8m3��
�ʴ�Ϊ��44.8��
| 32% | ||
|
��2�������ɶ������������Ϊm����
4FeS2+11O2=2Fe2O3+8SO2
4��120 8��64
120kg m
m=
| 120kg��8��64 |
| 4��120 |
�ʶ�����������ʵ���=
| 128��1000g |
| 64g/mol |
�ʴ�Ϊ��44.8��
���������⿼�黯ѧ����ʽ���йؼ��㣬�Ƚϻ�����ע�����⣨1����Ԫ�����������ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
���бȽ��У���ȷ���ǣ�������
| A���е�ߵͣ�HF��HCl��HBr��HI |
| B���뾶��С��F-��Cl-��Br-��I- |
| C����ԭ�Դ�С��Li��Na��K��Rb |
| D�����������٣�1H��2H��3H |
������ԭ��Ӧ�����ֻ������ͷ�Ӧ�Ĺ�ϵ��ͼ��ʾ�������л�ѧ��Ӧ��������3���ǣ�������
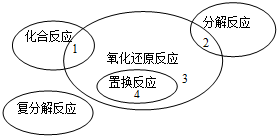

| A��4Fe��OH��2+O2+2H2O�T4Fe��OH��3 | ||||
B��2NaHCO3
| ||||
C��CH4+2O2
| ||||
| D��Cl2+2KBr�TBr2+2KCl |
�ڱ�״���£�11.2Lij�����������17g������������ǣ�������
| A��O2 |
| B��N2 |
| C��H2S |
| D��O3 |
 �ơ�þ������ͭ���仯�����ڿ��к������������й㷺��Ӧ�ã�
�ơ�þ������ͭ���仯�����ڿ��к������������й㷺��Ӧ�ã�