��Ŀ����
���³�ѹ�£��л���A�����ĺ����������е�Ψһһ����̬���ʣ�B��C��A�����ʽ��ͬ����������Է�������A��B��C��100��I�Ļ�ѧʽΪC4H4O4������֮�������ͼ��ʾת����ϵ���ش��������⣺
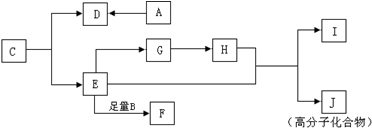
��1��A������Ϊ ��I�Ľṹ��ʽΪ ��
��2��Wg A��B�Ļ��������������ȫȼ�գ�������ͨ�������Ĺ������Ʒ�ĩ����ȫ���գ����徻�� g��
��3��E+B��F�Ļ�ѧ����ʽΪ ���÷�Ӧ����Ϊ ��
E+H��J�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
��4��6.0g��B��C�������ȫȼ������6.4g��������B��C��������Ϊ
A��1��1 B��1��2 C��2��1 D�������
��5��ij��������Է�������ΪB��2������һ�ȴ���ֻ������ͬ���칹�壬�����Ķ��ȴ��ﹲ�� ��ͬ���칹�壮

��1��A������Ϊ
��2��Wg A��B�Ļ��������������ȫȼ�գ�������ͨ�������Ĺ������Ʒ�ĩ����ȫ���գ����徻��
��3��E+B��F�Ļ�ѧ����ʽΪ
E+H��J�Ļ�ѧ����ʽΪ
��4��6.0g��B��C�������ȫȼ������6.4g��������B��C��������Ϊ
A��1��1 B��1��2 C��2��1 D�������
��5��ij��������Է�������ΪB��2������һ�ȴ���ֻ������ͬ���칹�壬�����Ķ��ȴ��ﹲ��
���㣺�л�����ƶ�
ר�⣺�л���Ļ�ѧ���ʼ��ƶ�
���������³�ѹ�£��л���A�����ĺ����������е�Ψһһ����̬���ʣ���AΪHCHO��B��C��A�����ʽ��ͬ����֪A��B��C�����ʽΪCH2O�������ǵ�ͨʽΪ��CH2O��n������������Է�������A��B��C��100���ɵ�30n��100����n=1��2��3����B��C�ķ���ʽ�ֱ�ΪC2H4O2��C3H6O3��I�ķ���ʽΪC4H4O4���ɷ����к�4����ԭ�ӣ��Ʋ�����Ƕ�Ԫ�������E��Hת����̼ԭ����û�б仯������E��H���Ӷ��Ǻ�2��̼ԭ�ӣ��ɴ���֪EΪ�Ҷ�����HOCH2CH2OH����GΪ�Ҷ�ȩ��OHC-CHO����HΪ�Ҷ��ᣨHOOC-COOH����IΪ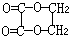 ��JΪ
��JΪ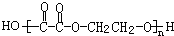 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽��
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽��
 ��JΪ
��JΪ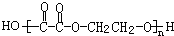 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽��
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬�ݴ˽�����
�⣺���³�ѹ�£��л���A�����ĺ����������е�Ψһһ����̬���ʣ���AΪHCHO��B��C��A�����ʽ��ͬ����֪A��B��C�����ʽΪCH2O�������ǵ�ͨʽΪ��CH2O��n������������Է�������A��B��C��100���ɵ�30n��100����n=1��2��3����B��C�ķ���ʽ�ֱ�ΪC2H4O2��C3H6O3��I�ķ���ʽΪC4H4O4���ɷ����к�4����ԭ�ӣ��Ʋ�����Ƕ�Ԫ�������E��Hת����̼ԭ����û�б仯������E��H���Ӷ��Ǻ�2��̼ԭ�ӣ��ɴ���֪EΪ�Ҷ�����HOCH2CH2OH����GΪ�Ҷ�ȩ��OHC-CHO����HΪ�Ҷ��ᣨHOOC-COOH����IΪ ��JΪ
��JΪ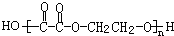 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬
��1��������������֪��AΪ��ȩ��I�Ľṹ��ʽΪ ���ʴ�Ϊ����ȩ��
���ʴ�Ϊ����ȩ�� ��
��
��2��A��B��ɵĻ����ijɷֿ��Ը�д�ɣ�nCO?mH2�������������ն�����̼��ˮ������Ӧ�仯Ϊ��Na2O2��Na2CO3��Na2O2��2NaOH���Ӿ������Ƕȷ������������ƾ����ա�CO���͡�H2����Wg A��B�Ļ��������������ȫȼ�գ�������ͨ�������Ĺ������Ʒ�ĩ����ȫ���գ����徻��wg���ʴ�Ϊ��w��
��3��E��B��Ӧ����B�����������ɶ�Ԫ����HOCH2CH2OH+2CH3COOH
CH3COOCH2CH2OOCCH3+2H2O������������Ӧ����ȡ����Ӧ����
E+H��J�Ļ�ѧ����ʽΪ��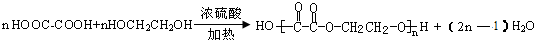 ���������۷�Ӧ��
���������۷�Ӧ��
�ʴ�Ϊ��HOCH2CH2OH+2CH3COOH
CH3COOCH2CH2OOCCH3+2H2O��������Ӧ����ȡ����Ӧ����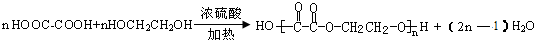 �����۷�Ӧ��
�����۷�Ӧ��
��4��B��C���ʽ��ͬ�������ǵ�̼���⡢����������ͬ��������B��C����̼���⡢��������ȣ����ԣ������Ժ��ֱ������B��C��һ���������������������һ������ѡ��D��
��5��BΪ���ᣬ��Է�������Ϊ60����÷���������Է�������Ϊ120������ʽΪC9H12����Ϊ����һ�ȴ���ֻ�����֣�������Ǹ߶ȶԳƣ�����������ֻ��1��3��5-���ױ������Ķ��ȴ����ͬ���칹�壬2����ԭ���ڼ�����2��ͬ���칹�壬2����ԭ���ڱ�������1��ͬ���칹�壬1����ԭ���ڱ����ϣ���1����ԭ���ڼ�����2��ͬ���칹�壬һ��5�֣��ʴ�Ϊ��5��
 ��JΪ
��JΪ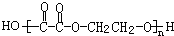 ��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬
��AΪ��ȩ��HCHO����BΪ���ᣨCH3COOH����DΪ���ᣨHCOOH����F���Ҷ��������������Ƶã�FΪ�������Ҷ�����CH3COOCH2CH2OOCCH3��������֪��C���Ҷ�����HOCH2CH2OH������ᣨHCOOH���������ɣ���1mol�Ҷ�����2mol������������CΪHCOOCH2CH2OOCH����Է�����������100������ʽΪC4H6O4��������C3H6O3����ȥ����1mol�Ҷ�����1mol������������CΪHCOOCH2CH2OH������ʽΪC3H6O3���������⣬��1��������������֪��AΪ��ȩ��I�Ľṹ��ʽΪ
 ���ʴ�Ϊ����ȩ��
���ʴ�Ϊ����ȩ�� ��
����2��A��B��ɵĻ����ijɷֿ��Ը�д�ɣ�nCO?mH2�������������ն�����̼��ˮ������Ӧ�仯Ϊ��Na2O2��Na2CO3��Na2O2��2NaOH���Ӿ������Ƕȷ������������ƾ����ա�CO���͡�H2����Wg A��B�Ļ��������������ȫȼ�գ�������ͨ�������Ĺ������Ʒ�ĩ����ȫ���գ����徻��wg���ʴ�Ϊ��w��
��3��E��B��Ӧ����B�����������ɶ�Ԫ����HOCH2CH2OH+2CH3COOH
| Ũ���� |
| �� |
E+H��J�Ļ�ѧ����ʽΪ��
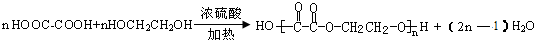 ���������۷�Ӧ��
���������۷�Ӧ���ʴ�Ϊ��HOCH2CH2OH+2CH3COOH
| Ũ���� |
| �� |
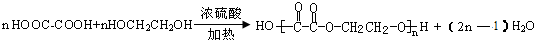 �����۷�Ӧ��
�����۷�Ӧ����4��B��C���ʽ��ͬ�������ǵ�̼���⡢����������ͬ��������B��C����̼���⡢��������ȣ����ԣ������Ժ��ֱ������B��C��һ���������������������һ������ѡ��D��
��5��BΪ���ᣬ��Է�������Ϊ60����÷���������Է�������Ϊ120������ʽΪC9H12����Ϊ����һ�ȴ���ֻ�����֣�������Ǹ߶ȶԳƣ�����������ֻ��1��3��5-���ױ������Ķ��ȴ����ͬ���칹�壬2����ԭ���ڼ�����2��ͬ���칹�壬2����ԭ���ڱ�������1��ͬ���칹�壬1����ԭ���ڱ����ϣ���1����ԭ���ڼ�����2��ͬ���칹�壬һ��5�֣��ʴ�Ϊ��5��
���������⿼���л����ƶϣ�����ȷ��B��C����ʽ���ٸ���ת����ϵ��I�ķ���ʽ�²���Ϊ���������ƶ��������ʣ��Ƕ��л���ѧ���ۺϿ��飬��Ҫѧ���������չ����ŵ�������ת�����ϺõĿ����γɷ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
 ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
ֱͨ������У�ܲ��¿�ֱͨ��Уϵ�д�
�����Ŀ
���и���Һ�У�pH�����ǣ�������
| A��pH=9�İ�ˮ��Һϡ��1000�� |
| B��pH=9���ռ���Һϡ��1000�� |
| C��pH=5��������Һϡ��1000�� |
| D��pH=5���Ȼ����Һϡ��1000�� |
����������������ż��䵥λ�ı��������ǣ�������
| A��Ħ������ M g/mol |
| B������Ħ����� Vm L/mol |
| C���ܽ�� S g/100g |
| D���ܶ� �� g/cm3 |
���з�Ӧ�У��������ӷ���ʽH++OH-=H2O��ʾ���ǣ�������
| A������������������Һ |
| B��ϡ����������������Һ |
| C������������Һ������������Һ |
| D�������백ˮ |
���������缫����KOH��Һ�У��������ֱ�ͨ��CH4��O2�����ɼ���ȼ�ϵ�أ���֪��ͨ��CH4��һ������缫��Ӧʽ�ǣ�CH4+10OH--8e-=CO32-+7H2O��ͨ��O2����һ������缫��Ӧʽ�ǣ�2O2+4H2O+8e-=8OH-������������ȷ���ǣ�������
| A��ͨ��CH4�ĵ缫Ϊ���� |
| B���õ��ʹ��һ��ʱ���Ӧ����KOH |
| C����������������Ӧ |
| D��ȼ�ϵ�ع���ʱ����Һ�е�OH-�������ƶ� |

 �ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��
�ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ�� Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ����¡�Fe��ˮ�����ķ�Ӧʵ�顱��